INTRODUCTION
Over the past several decades, there is a global surge in chronic kidney disease (CKD).1,2In industrialized countries, with the control of communicable diseases and treatment-associated reduction in cardiovascular events, CKD has become a major contributor to health burden associated with high economic cost to health systems.3,4 In contrast, in developing countries, where communicable disorders have not been completely eradicated, the surge of chronic renal impairment in the population is an additional factor to increased cost to healthcare.4,5
Several epidemiologic surveys and clinical studies have demonstrated that CKD is a major independent determinant risk for cardiovascular disease leading to a further increase in economic cost to healthcare.3-6
DEFINITION OF CHRONIC KIDNEY DISEASE
CKD is defined as abnormalities of kidney structure and or function persistent for more than 3 months.1
The indicators of kidney damage include: (Table 1)
| Table 1. Indicators of Kidney Damage |
1- Imaging abnormalities
2- Reduced glomerular filtration rate
3- Increased urinary protein/albumin excretion (Proteinuria/albuminuria)
4- Urinary sediment abnormalities
5- Kidney transplant recipients |
Imaging Abnormalities
By various different imaging techniques, diseases of renal structure vessels and or collecting systems can be detected.7 However, the presence of simple kidney cysts is not generally diagnostic of CKD.7
Decreased Renal Function
Glomerular filtration rate (GFR) is widely recognized as the best overall index of kidney function.7 Reduction in GFR is generally associated with widespread damage of kidney tissues and decline in other kidney functions in CKD.7
Measurement of GFR by determination of external filtration markers such as inulin is cumbersome and unpractical.1
Values of GFR are estimated on the basis of plasma concentration of creatinine. The preferred equation to estimate GFR (eGFR) is that of the Chronic Kidney Disease Epidemiology Collaboration (commonly known as the CKD-EPI) which takes into account sex, age, ethnic origin and serum creatinine concentration.1 However, plasma creatinine concentrations may be affected by some factors, including creatinine generation, tubular secretion and extrarenal excretion (Table 2) which may lead to possible variations between populations.1
| Table 2. Determinants of Serum Creatinine Concentration |
1- Creatinine generation
– Muscle mass
– Dietary intake |
2- Excretion
– Glomerular
– Tubular secretion
– Extrarenal removal |
CKD-EPI replaces the Modification of Diet Renal Disease (MDRD) equation as a more accurate predictor of renal risk.1
A threshold of GFR <60 ml/min/1.73 m² for >3 months has been selected to indicate CKD as this value represents less than 50% of the normal GFR value of 125 ml/min/1.73m² of young men and women.7
A GFR <60 ml/min/1.73 m² can be detected by routine laboratory tests. The current estimating equations for GFR (eGFR) based on plasma creatinine, but not plasma creatinine alone are sensitive for detecting measured GFR <60 ml/min/1.73 m².7
The risk of cardiovascular disease (CVD) is greater in subjects with CKD and GFR <60 ml/min/1.73 m² than in subjects with preserved GFR.7
GFR <60 ml/min/1.73 m² indicates a reduced GFR while <15 ml/min/1.73 m² represents renal failure.8
Proteinuria/Albuminuria
Proteinuria:
a term which refers to increased urinary protein excretion may result from several mechanisms:7
a. Glomerular proteinuria/albuminuria caused by increased glomerular permeability to large molecular weight (MW) proteins.
b. Tubular proteinuria due to incomplete tubular reabsorption of normally filtered low MW proteins.
c. Overproduction proteinuria from increased plasma concentration of low MW proteins such as immunoglobulin light chains.
d. Renal tubular cell proteinuria caused by abnormal loss of renal cell constituents secondary to tubular damage.
Proteinuria is determined by a urine protein to creatinine ratio (mg/mg) with abnormal proteinuria representing a ratio ≥0.2 mg/mg which corresponds to 250 mg/24 hours.3
Albuminuria, tubular proteinuria and renal tubular cell proteinuria are highly suggestive of kidney damage.7
Albuminuria:
This refers to increased urinary excretion of albumin.7
Recent guidelines recommend changing the focus from proteinuria to albuminuria as i) albumin is the major component of urinary protein in most CKD and ii) as a graded relationship exists between amount of urinary albumin and risk of both kidney and cardiovascular diseases.7
Albuminuria is a common finding in CKD. In diabetic glomerulosclerosis, albuminuria precedes any reduction in GFR; while in hypertensive nephrosclerosis, it is often detected after the development of renal functional impairment.7 Albuminuria has also been reported in some cases of hypertension, obesity and cardiovascular diseases, though the underlying renal pathology has not been defined in these situations.7
The rate of loss of urinary albumin is commonly referred to albumin excretion rate (AER).7
A threshold of AER (albumin excretion rates) ≥30 mg/24 hour (which is equivalent to an albumin-creatinine ratio (ACR) of ≥30 mg/g or ≥3 mg/mmol in a random untimed urine sample) has been selected to indicate CKD for the following reasons:7
a. An AER ≥30 mg/24 hrs (ACR ≥30 mg/g or ≥3 mg/mmol) is greater than 3 times the normal AER value of 10 mg/24 hrs (ACR=10 mg/g or 1 mg/mmol) in young adult men and women.7
b. An AER ≥ 30 mg/24 hrs (ACR ≥ 30 mg/g or ≥3 mg/mmol) is associated with increased risk of all-cause and cardiovascular mortality, renal failure, acute kidney injury and progression of CKD.7
Urinary Sediment Abnormalities
Renal tubular cells, red blood cells (RBC) casts, white blood cells (WBC) casts, coarse granular casts, wide casts and dysmorphic RBC are indicative of kidney damage.7
Kidney Transplant Recipients
Such patients are considered to have CKD independent of the level of GFR and or presence of markers of kidney damage.7 Kidney biopsies in such patients often reveal pathologic evidence of CKD.
PREVALENCE AND INCIDENCE
CKD is a frequent disorder. Several surveys and clinical studies reveal a high global prevalence of 11-13%.1,9 However, prevalence and incidence rates differ across countries and regions (Table 3).9- 12 In 2002-2003, higher prevalence rates of CKD of 16 and 14% were reported in USA in NHANES III ( 3rd National Health and Nutrition Survey) and in Australia in AUS DIAB (the Australian diabetes, obesity and lifestyle study) respectively.13,14 Over 80% of all patients who receive treatment for end stage kidney disease are reported from developed countries which are characterized by a large elderly population and access to healthcare facilities.1 The annual incident rate of renal replacement therapy is much higher in affluent countries, ranging from 150 to 400 per million population (pmp) compared to 50 pmp in poor countries with limited access to healthcare.9 Further within countries, certain population subgroups appear to be at higher risk of CKD.1
| Table 3. Prevalence of Chronic Kidney Disease in Various Continents/Countries9-12 |
|
Continents/Countries
|
Prevalence Rate (%)
|
| • Global |
11-13 |
|
| • Asia |
|
| # East Asia |
|
* Thailand
* Malaysia
* China |
18
10
10-19 |
| # South East Asia |
|
* Bangladesh
* India
* Pakistan |
>20 |
* Nepal
* Srilanka |
10-20 |
| * South Korea |
|
| – Men |
2.6 |
| – Women |
4.6 |
| • Africa |
|
| # Subsaharan Africa |
|
* Senegal
* Ghana
* Democratic Republic Congo
* Tanzania |
5-17 |
| • America |
16 |
| # USA |
|
| #Latin America |
|
* Mexico
*El Salvador
* Guatemala
* Costa Rica
* Honduras
* Nicaragua
* Belize |
15-20 |
| • Middle East |
|
| # Iran |
5-23 |
| # Turkey |
16 |
| # Arab Countries |
|
*Saudi Arabia
* Egypt
* Lebanon
* Kuwait |
25
35
46.8
21.1 |
Related to diabetic nephropathy
|
| • Europe |
|
|
| # Norway |
11.1 |
| # England |
5.2 |
| # Germany |
25.6 |
| • Australia |
14 |
CKD appears to be more common in women than men, but the cause of this observation remains elusive.15 Further, estimates indicate that CKD may be even higher than diabetes mellitus, with an estimated prevalence of 8.2% in the latter.
The prevalence of CKD is increasing worldwide in both affluent and developing countries. Data from the American National Health and Nutrition Examination Survey revealed that the prevalence of CKD rose significantly from 10 to 13.1% in the periods 1988-1994 to 1999-2004.1 Similar observations have been reported in developing countries such as China and India.1
Demographics of patients with CKD differ widely between countries. For example, patients with CKD are younger in India than in China (51 vs 63.1 years) with a predominance of an undetermined etiology.1
CLASSIFICATION AND STAGES OF CKD
Using the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines, CKD is classified into 5 stages based on decreasing GFR values and 3 categories of increasing albuminuria (Table 4) and (Figures 1 and 2).7,16 Further, the National Institute for Health and Care Excellence (NICE) guidelines recommend to further subdivide stage 3 into 3A and 3B, based on increasing CVD risk associated with greater functional renal impairment (Figures 2 and 2A).17
| Table 4. Classification and Staging of Chronic Kidney Disease.7 |
|
GFR Category
|
GFR value (ml/min/1.73m²)
|
|
|
|
G I
|
>90 |
|
|
|
G II
|
60-89
|
|
|
|
G III
|
|
|
|
| A |
45-59
|
|
|
|
B
|
30-44
|
|
|
|
G IV
|
15-29
|
|
|
|
G V
|
<15
|
|
|
|
Albuminuria Category
|
AER (mg/24hr) |
ACR equivalent |
| mg/mmol |
mg/g
|
|
A1
|
<30 |
<3 |
<30
|
|
A2
|
30-300 |
3-30 |
30-300
|
|
A3
|
>300 |
>30 |
>300
|
| AER: Albumin Excretion Rate, ACR: Albumin-to-Creatinine Ratio, GFR: Glomerular Filtration Rate |
Figure 1. Classification and Prevalence of Chronic Kidney Disease Stages.4 CKD: Chronic Kidney Disease, ACR= Albumin/ Creatinine Ratio
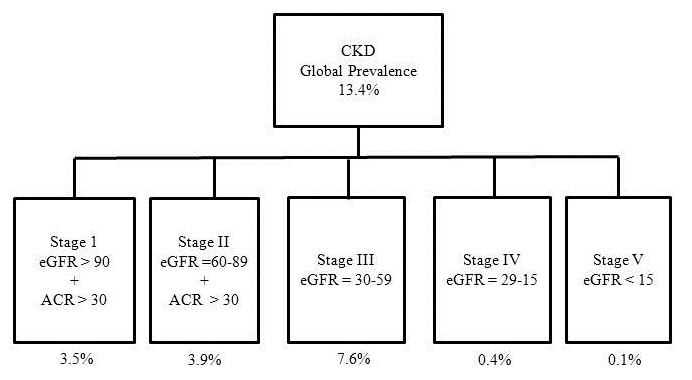
Figure 2. Classification of Chronic Kidney Disease
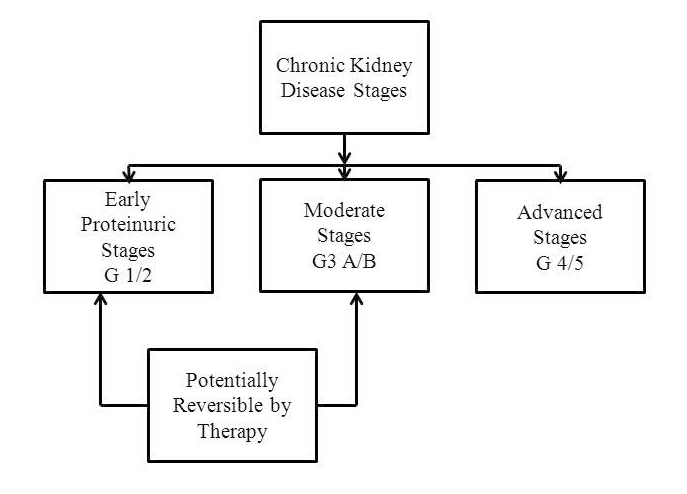
Figure 2A. Classification of Chronic Kidney Disease Stage 3
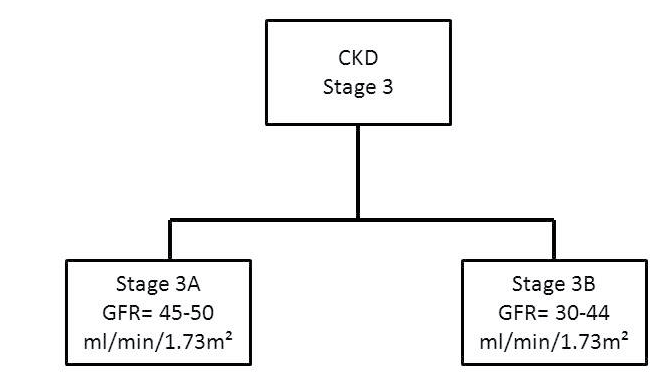
In a UK study, it has been estimated that stage 3 represents about 90% of CKD cases, with 84% and 16% reflecting stages 3A and 3B respectively.18
ETIOLOGY AND RISK FACTORS
Several factors underlie CKD (Figure 3).
Figure 3. Etiology of Chronic Kidney Disease
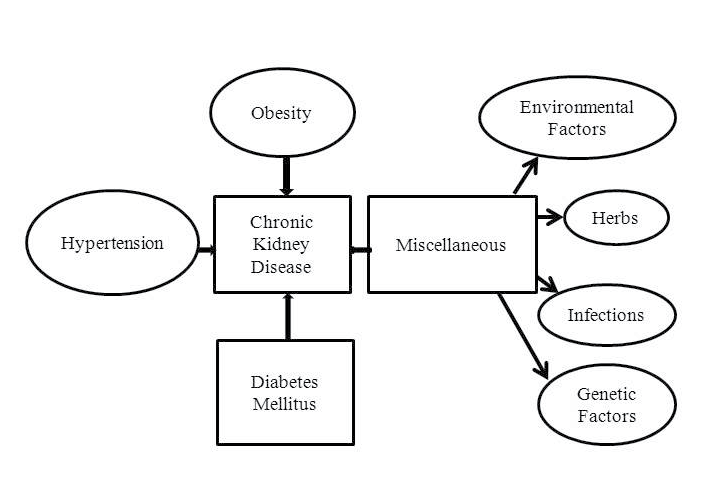 In affluent and several developing countries, abnormal lifestyle approaches play an important role in the development of CKD.1 Hypertension, diabetes mellitus and obesity are both the major cause and risk of CKD.1 Further, increased consumption of carbonated beverages, high salt intake and smoking contribute to the burden of chronic nephropathy.19,20
In affluent and several developing countries, abnormal lifestyle approaches play an important role in the development of CKD.1 Hypertension, diabetes mellitus and obesity are both the major cause and risk of CKD.1 Further, increased consumption of carbonated beverages, high salt intake and smoking contribute to the burden of chronic nephropathy.19,20
In many low income countries, inadequate eradication of communicable disorders, infections, glomerulonephritis, environmental toxic products, poor sanitary facilities, analgesic abuse, herbal medicines, pesticides, use of contaminated water remain important etiologic determinants of renal functional impairment (Figure 3).1,21,22
Glomerulopathies, especially those associated with high grade proteinuria, are frequent causes of CKD, accounting for about 18% of patients receiving renal replacement therapy programs.23,24 Proteinuric glomerulonephritis, generally classified into inflammatory and non-inflammatory disorders include post streptococcal glomerulonephritis, lupus nephritis, focal and segmental glomerulosclerosis, membranous nephropathy and small vessel vasculitides.23 Hypertension frequently complicates these diseases and contributes to progression to renal failure and cardiovascular conditions.23
Balkan nephropathy, a type of progressive tubulointerstitial nephropathy leading to end-stage renal disease is attributed to the consumption of herbs containing Aristolochic acid.25,26 Similar cases have been reported in Taiwan and China.1
Genetic factors appear to be contributors. Variations in Myosin heavy chain 9 (MYH9) and Apolipoprotein L1 (APOL1) are associated with non-diabetic chronic nephropathy in subjects of African origin.1,27,28
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary renal disorder that leads to end stage renal disease (ESRD), which accounts, with other cystic kidney diseases for 5% of patients entering dialysis programs.24,29
The cause of the illness is determined by the presence of PKD1 or PKD2 genes.29 A correlation has been demonstrated between a change in total kidney volume (TKV) and reduction in glomerular filtration rate (GFR).29 Although cysts grow progressively over time, the development of CKD lags behind the growth of the renal cysts.29 Progressive renal failure develops in the 5th decade of life.29 Women tend to have smaller kidneys than men, and thus develop ESRD at a later stage.29
ADPKD is associated with a significant number both, of renal and extra-renal manifestations.29 These include hypertension, infections, nephrolithiasis, pain related to cysts, space occupying masses, cerebral aneurysms, cysts in other target organs, and diverticular disease.29,30
Hypertension without any demonstrable renal impairment occurs in over 60% of ADPKD patients and contributes to cardiovascular diseases.30
Cardiovascular complications are the most frequent causes of death.29,30 Autopsy studies reveal severe left ventricular hypertrophy and coronary artery disease.30 Cardiovascular abnormalities often start in childhood.30
OUTCOME AND COMPLICATIONS
CKD is known to be associated with increased morbidity, mortality and utilization of healthcare facilities.8
Several general population cohort studies have reported significantly reduced life expectancy in patients with CKD (Table 5).31 In a Canadian general population cohort, in patients aged 30 years with an eGFR of 30-44 ml/min/1.73 m² (stage 3B) and 15- 29 ml/min/1.73 m² (stage 4), life expectancy was reduced by 17 and 25 years respectively compared with subjects with normal renal function.6,32 Similarly, in the same study, presence of albuminuria was associated with reduced life expectancy: stage 2 (albuminuria: 30-299 mg/gcr) and stage 3 (albuminuria >300 mg/gcr) life expectancy was shortened by 10 and 18 years respectively.6,32 In contrast, in middle-aged subjects, diabetes mellitus or hypertension decreased life expectancy by only 8 and 2-3 years respectively (Table 5).6,33,34
| Table 5. Life Expectancy Reduction in CKD, Diabetes, Hypertension |
| |
Clinical Entity
|
Reduction in life expectancy (years)
|
|
1- Chronic kidney disease
|
|
GFR(ml/min/1.73m²)
|
|
Stage 3B
|
30-44 |
17 |
| Stage 4 |
15-29 |
25
|
|
Albuminuria (mg/gCr)
|
|
Stage 2
|
30-299 |
10 |
| Stage 3 |
300 |
18
|
| |
2- Hypertension
|
2-3 |
| |
3- Diabetes Mellitus |
8
|
Similar observations were reported in other general population studies.35
CKD is a major risk for cardiovascular disease (CVD).6 CKD patients tend to die from CVD outcome than to progress to end stage renal failure.6,36 In NHANES II, an estimated glomerular filtration rate (eGFR) of less than 70 ml/min/1.73 m² was associated with 68% and 51% increases in risk of death from any cause and from CVD respectively compared with eGFR of at least 90 ml/min/1.73 m².8
Studies in various population cohorts have evaluated the influence of low eGFR and the increased urinary albumin excretion on CVD mortality. Data from several cohort studies involving about 1.4 million subjects were assessed in meta-analyses after adjustment for traditional CVD risk factors and albuminuria.6,36 The relationship between eGFR and CVD mortality was nonlinear (Table 6).6,36 When eGFR was higher than 75 ml/min/1.73 m², there was no change in risk gradient in CVD mortality (Table 6).6,36 However, an increment in CVD risk mortality was observed with decreasing eGFR. CVD mortality was twice as high in patients with stage 3 CKD (eGFR=30-59 ml/min/1.73 m²) and 3 times higher in stage 4 (eGFR=15-29 ml/min/1.73 m²) compared to subjects with normal renal function (Table 6).6,36 In contrast, the association of albuminuria with risk of CVD death appears to have no threshold and is linear.6,36 The adjusted risk of CVD mortality started to increase at a threshold of albuminuria of ≥ 30 mg/g and was twice as high in the albuminuria range (stage 2) of 30-299 mg/g compared to the risk in subjects with normal albuminuria (Table 6).6,36
| Table 6. Chronic Kidney Disease–Cardiovascular Disease Mortality Relationship.6 |
|
Estimated GFR
(ml/min/1.73 m²)
|
CKD-CVD
Relationship |
CVD
Mortality risk
|
|
>75
|
Nonlinear |
Normal |
| 30-59 (stage 3) |
Linear |
Increased by 2 times
|
|
15-29
|
Linear |
Increased by 3 times |
|
Albuminuria (mg/g)
|
|
|
|
>30-299
|
Linear |
Increased by 2 times
|
A large number of cardiovascular disorders complicate the course of CKD. Compared to subjects with preserved renal function, subjects with eGFR <60 ml/min/1.73 m² are at increased risk of atrial fibrillation, coronary artery disease, heart failure, stroke, cognitive impairment, peripheral arterial disease, and all cause and cardiovascular mortality.6,8,17,37,38 In addition, CKD patients tend to develop anemia, mineral and bone disorders, fracture, and a poor quality of life.37
Both early stages of CKD and end stage renal disease are associated with high morbidity and increased healthcare utilization.39 The prevalence of comorbid conditions is similar in both early and late stages of CKD: cardiovascular disease (40 vs 59%), cerebrovascular disease (12 vs 8%), and peripheral arterial disease (14 vs 14%).40
PATHOPHYSIOLOGIC MECHANISMS
Mediators that link the association between CKD and CVD have not been completely elucidated but appear multifactorial. Several studies indicate that the increased CVD risk in CKD is due to a combination of traditional and nontraditional kidney specific factors.6,37
Hypertension and diabetes mellitus, well known risks for CVD, play an important role in the development of CKD in about 50% of patients.1,6 However, in non-diabetic, non-hypertensive patients, CKD still confers a high CVD risk.6 In one study, CKD superseded coronary risk conferred by diabetes mellitus.41
An atherogenic lipid profile is a frequent metabolic abnormality in CKD. The dyslipidemia, characterized by defective HDL-cholesterol and excessive oxidation of LDL-cholesterol, is a major contributor to CVD risk.6
Several cardiovascular clinicopathophysiologic entities, specific to CKD, are frequently observed in early and advanced stages of renal impairment.6 Left ventricular hypertrophy (LVH), predominantly of the concentric type, is highly prevalent, being reported in about 50% of CKD patients with eGFR <30 ml/min/1.73 m².6,42,43 Left ventricular hypertrophy (LVH) expresses reduced coronary reserve, myocardial fibrosis, reduced cardiac capillary density, down-regulation of nitric oxide synthase system and impaired endothelial function, leading to impaired coronary dilatation and myocardial contractility and disturbances in cardiac rhythm.6,42,43,44
Anemia, hypertension and increased vascular stiffness play a major role in the genesis of LVH.6,43
The association of CKD with the several kidney specific cardiovascular disorders is termed cardiorenal syndrome.45
Besides LVH, the high prevalence of coronary artery disease and electrolyte imbalance account for the significant cardiovascular morbidity and mortality and sudden cardiac death in CKD.6,43
Modifications in the humoral vasoactive system have been reported to contribute to the heightened CVD risk in CKD. Nitric oxide (NO) system, involved in endothelial function, vascular smooth muscle contraction and growth, platelet aggregation and leukocyte adhesion to endothelium, is impaired.6 Bioavailability of NO is reduced, at least partly, due to increasing concentrations of asymmetric dimethyl arginine associated with impaired renal function.6,46 Deficient generation of NO leads to reduced cardiac output, increased systemic vascular resistance and BP and left ventricular hypertrophy.6,46 Activity of renalase, an enzyme produced by the kidney and involved in inactivation of catecholamines, is reduced, exacerbating overactivity of the sympathetic nervous system, frequently observed in CKD.6
Enhanced activity of the renin-angiotensin and sympathetic nervous systems also contribute to CVD risk. Increased angiotensin stimulates production of superoxide, interleukin 6 and other cytokines.6
Low grade systemic inflammation is a frequent manifestation in CKD.6 It is involved both as a promoter of CVD and in progression of CKD.6,44 Low grade systemic inflammation is attributed to the production of inflammatory markers leading to oxidative stress and accumulation of modified proteins and toxins that are cleared by a normally functioning kidney.44
Systemic atherosclerosis and calcific valvular heart disease may complicate the course of both early and late stages of CKD.6,44 Several factors have been postulated to modulate these complications, including calcification inhibitors (fetuin-A and matrix Gla protein), and promoters (hyperphosphatemia), disturbed calcium-phosphate product, parathyroid hormone and leptin.6,47
Deficiency of active vitamin D commonly observed in CKD, is associated with increased risk of CVD.6 It is due to lack of a precursor or impaired activity of a kidney enzyme one alpha hydroxylase, required converters to the active compound.48
PREVENTIVE AND THERAPEUTIC APPROACHES
Effective strategies can prevent and slow progression of CKD and reduce the risk of CVD.1
Although the etiology of CKD is multifactorial, diabetes mellitus and hypertension remain the major causes of renal functional impairment and end-stage renal disease.1,6 Further, recent studies indicate that obesity is becoming an important risk for development of CKD.1,21
Preventive measures include awareness and screening.
Awareness of CKD
Although it is well established that it is an important public health burden, awareness of CKD both by the general population and healthcare providers is poor. In a nation-wide health screening program in USA, involving about 90000 adults at high CKD risk, the prevalence and awareness rates were respectively 29.7% and 8.6% for Caucasians, 22.8% and 6.3% for Afro-Americans, 29.2% and 6.8% for Native Americans, 20.3% and 11.1% for Hispanics, and 23.4% and 11.9% for Asians and Pacific Islanders.1,37 Similar low awareness rates were reported in China and Taiwan.1
Healthcare providers also do not realize the prognostic significance of CKD. In a nationwide audit study in Italy involving about 450000 adults followed-up by general practitioners, only in 17% was serum creatinine determined, of whom 16% had GFR lower than 60 ml/min/1.73 m².1 Among these adults, CKD was correctly diagnosed only in 15%.1 In another study which includes about 40000 hypertensive patients, 23% had CKD but the condition was diagnosed only in 3.9% by the general practitioners.1,49
Screening
Identification of chronic kidney disease (CKD) relies on measurements of serum creatinine, glomerular filtration rate and or albuminuria.1 However, the most appropriate cost-effective approach to screen for CKD remains unclear.1The National Kidney Foundation recommends screening individuals with diabetes mellitus, hypertension, cardiovascular disease, structural renal disease, autoimmune disease with risk of kidney involvement and family history of kidney disease (Table 7).7,16
| Table 7. Screening Recommendations for Kidney Disease.6 |
• Diabetes
• Hypertension
• Cardiovascular disease
• Structural diseases of renal tract
• Autoimmune diseases for kidney involvement potential
• Family history of kidney disease |
Studies revealed that screening for CKD appears to be cost effective in diabetes, particularly in those older than 50 years of age.1 Further, administration of renin-angiotensin system blockers to proteinuric diabetics improved both cost effectiveness and life expectancy and reduced the cumulative incidence of end stage renal disease.1,50
Low economic status appears to be associated with increased risk of CKD.1 In the 3rd National Health and Nutrition Examination Survey, poverty was linked to an increased risk of albuminuria independent of other influences such as hypertension, obesity, low glomerular filtration rate and use of medications.1
Preventive and Therapeutic Measures
Prevention and or delay in CKD progression require adequate: a) glycemic regulation and b) BP control. Any class of antihypertensive medications can be used, but blockers of the renin-angiotensin system are preferred due to their albuminuria reducing action; however, with their use, serum creatinine and serum potassium should be frequently monitored.6 Long-acting diuretics may be indicated in the presence of moderate to severe renal functional impairment.6 It is essential to achieve appropriate salt and protein intake.6
Initiation of a combination of angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB) in ADPKD patients appears to be effective in strict BP control and in slowing cyst progression.29,30 In patients with normal renal function, a high fluid intake is an additional therapeutic measure that may delay cyst growth.30
Lipid lowering drugs are indicated for the treatment of dyslipidemia and CVD protection.6 However, there is no evidence that they prevent or delay CKD progression.6
Recent evidence suggests that a Mediterranean Diet is associated with both renoprotection and improved survival.51 The relation between adherence to a Mediterranean dietary pattern, renal function and mortality was assessed in a population-based cohort of 1111 elderly Swedish men (age=70 years).51 Greater adherence to the Mediterranean Diet predicted a lower risk of CKD and greater 10 year survival in those with manifest CKD.51,52 Similarly, in an international study involving elderly subjects at high risk of coronary heart disease, a Mediterranean diet, maintained for one year, appears to be associated with an improvement in renal function.53
RELATION BETWEEN ACUTE KIDNEY INJURY (AKI), CHRONIC KIDNEY DISEASE (CKD) AND END-STAGE RENAL DISEASE (ESRD)
Recent evidence suggests that AKI can enhance the risk of CKD and End-stage renal disease (ESRD).
AKI, a common complication of critically ill patients, occurs in up to 20% of hospital admission.54 AKI is defined as an abrupt reduction in renal function leading to retention of nitrogenous waste products and disturbances in the extracellular volume and serum electrolytes.55
According to the KDIGO guidelines, the criteria for AKI include one or more of the following features55: an increase in serum creatinine by ≥0.3 mg/dL within 48 hours, a rise in serum creatinine to ≥1.5 times baseline, occurring within the past 7 days and or reduced urine value <0.5 mg/kg/hour for six hours.55
Several clinical and experimental studies indicate that patients who survive AKI are at greater risk for CKD, ESRD and other outcomes related to non-renal target organ involvement.54,56 The risk for CKD and ESRD increases in a graded fashion from mild to severe AKI.56
The mechanism (s) by which AKI enhances the risk for CKD, ESRD and non-renal target organ damage remain(s) elusive. Observations in experimental animals indicate that AKI can cause renal fibrosis and involve other target organs including lungs, heart and liver.56
CONCLUSION
CKD, defined as abnormal structure and or function of the kidney persisting for 3 months is a highly prevalent worldwide condition associated with significant risk of cardiovascular morbidity and mortality. Most patients with CKD die of cardiovascular events rather than progressing to end stage renal disease. Based on glomerular filtration and urinary albumin excretion rates, CKD is classified into 5 and 3 stages respectively. Diabetes mellitus, hypertension, and obesity are the most frequent causes although other mediators such as herbs, medications, genetic factors and poor lifestyle measures may be involved. Increased awareness of the health burden of renal functional impairment, proper lifestyle measures including sports, weight reduction, salt restriction, and adequate glycemic and BP control can prevent the development and or progression of CKD.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.




 In affluent and several developing countries, abnormal lifestyle approaches play an important role in the development of CKD.
In affluent and several developing countries, abnormal lifestyle approaches play an important role in the development of CKD.



