INTRODUCTION
The Medical Radiation Practice Board of Australia’s professional capabilities (Domain 5), state that registered radiographers must “provide safe radiation practice” by ensuring that the likelihood of exposure is kept as low as reasonably achievable.1 The three principles that enforce this are to minimise time, maximise distance and apply shielding.2
Radiographers abide by these principles because of stochastic and deterministic effects associated with radiation. Stochastic effects of ionising radiation are chance events, such as cancer and genetic effects, with the probability of the effect increasing with increasing radiation dose.2 There is no threshold associated with it and the effects are most likely to occur later in life. A deterministic effect occurs once a threshold is reached, and the biological response of the effect increases with radiation dose.2
Lead has a high atomic number (Z=82) and a high atomic mass (A=208) which makes it an effective shielding material from X-Ray photons.3 Radiation personal protective equipment are composed of lead, and these include gonadal shields, lead aprons and thyroid shields. Gonad shielding was introduced in 1905 to prevent male sterility and became more prevalent in the 1950s as hereditary risks became a major concern after atomic bomb survivors exhibited high cancer incidence.4,5 Since then, they have been used to protect reproductive organs from radiation. However, subsequent generations of atomic bomb survivors have not displayed any signs of radiation induced genetic effects or having increased risks to other diseases.6 Epidemiological studies of the atomic bomb survivors also recognised the higher risk of inducing cancer in children compared to adults, specifically when exposed to ionising radiation in early childhood. Furthermore, children are 3-4 times more radiosensitive than adults due to their longer life expectancy and many cancers remain latent.7
Currently, there are variations in the use of gonadal shields amongst radiographers.8,9,10 In Hayre et al8 ethnographic study, this inconsistency was attributed to “word of mouth” or to protect the “patient’s well-being”. Shanley et al9 found that 57% of radiography educators teach students to apply gonadal shielding and only 22% taught students to apply shielding to breasts, despite knowing that breast tissue is more radiosensitive than gonads. An Australian study showed differing radiographers’ attitudes to applying gonad shields. Several professional bodies have recently released positional statements in support of ceasing the use of gonadal shields. This was first released by the American Association of Physicists in Medicine (AAPM), followed by the Australian Society of Medical Imaging and Radiation Therapy (ASMIRT) and the National Council on Radiation Protection (NCRP).11,12,13
This literature review explores the risks and benefits of using lead protection and discusses the current state of the use of gonadal shields in clinical practice.
METHODS
A search of the literature was conducted through Ovid Medline, PubMed, and Scopus databases under the subject “Medical Imaging and Radiation Sciences”, which allowed access to. Google Scholar was also another database that was utilised. A combination of search methods was used such as combining key terms and phrases, phrase searching, truncation and exploding Medical Subject Headings (MeSH). Key terms and phrases were combined using Boolean operators “AND” to link key concepts as well as “OR” for synonyms of key concepts. For example, for the key concepts “lead protection” and “plain film imaging”, the relative key words used were “lead shielding” OR “gonadal shielding” AND “X-ray” OR “radiography” OR “pelvic radiography”. In PubMed, “radiation protection” was searched under the MeSH database, the subheadings “methods” and “I” were selected then this was combined with the phrase “gonadal shielding” using “AND”. Articles pertaining to radiation therapy and those not in English were excluded from the review. Only original research articles were included in the review, reviews of the literature were excluded, other than for background. The relevant articles were downloaded into endnote and reviewed.
DISCUSSION
Risks of Using Lead Protection in Conventional Imaging
Low benefit seen in gonad shielding:
Tissue weighting factor (Wt ) measures the risk of stochastic effect from irradiation of specific tissues or organs.2 In 1997, the International Commission on Radiological Protection (ICRP) stated that the Wt of the gonads was 0.25. In 1990, this decreased to 0.20 and since 2007 the Wt had diminished further to 0.08.14,15,16 In comparison to breast tissue, lung, stomach, colon and bone marrow which has a Wt of 0.12, the gonads are less radiosensitive.15 Interestingly, one study revealed that radiography educators agreed that lead shielding should be used for highly radiosensitive organs however, disagreed that lead shielding is more important for the colon than the gonads9 which displays a lack of currency amongst radiography educators.
In conjunction with the reduced Wt , technological advancements such as highly sensitive image receptors and digital image processing, have contributed to optimised X-ray systems that reduces radiation dose in pelvic X-ray examinations.4,5,17 Kemerink et al17 reconstructed the entrance surface air kerma (ESAK) for radiation doses prior to 1927, and found that the radiation dose for pelvic examinations has reduced by a factor of 400 from 1896 to 2018 (Figure 1).17 Frantzen et al5 stated that the dose for a pelvic radiograph in the 1950s was around 10 mGy whereas now it is in the range of a tenth of a mGy. These advances in technology have fuelled the calls for the discontinuation of gonad shields in removing outdated practice.
Frantzen et al5 found that gonadal shielding reduced hereditary risk for children between the ages of 0-15-years by 6±3% and 24±6% for girls and boys, respectively. However, the inherently low hereditary risk without shielding between 0.1×10-6 to 1.3×10-6 for females and 0.3×10-6 to 3.9×10-6 for males makes it questionable whether the risk of losing diagnostic information, repeating X-rays, and the possibility of altering the automatic exposure control (AEC) chambers are worth taking. Effective dose is a quantitative measurement which takes into consideration the type of organ and radiation, to give an indication of stochastic risk.16 The effective dose found in their study for pelvic examinations were between 0.008 to 0.098 mSv, which could be considered negligible. In Australia, the general public is exposed to natural background radiation which is equivalent to 1.5 mSv per year.18 A typical adult dose resulting from a pelvic X-ray is 0.6 mSv, which is equivalent to 5-months of background radiation dose.19 The usefulness of gonad shields is questionable as the radiosensitivity of the gonads is relatively low, current operating systems do not produce high doses compared to previously, and there is a risk of obscuring important anatomy.14,20
Inaccurate placement of gonadal shields:
Most articles had similar criteria to what is considered accurate or inaccurate shielding. For males, the ideal position of the gonad shield appears below the pubic arch and covers the testes.21-25 In females this occurs when the shield is seen above the pubic symphysis, next to the ischial spines and covering the pelvic contents within the pelvic ring.21-26 It was found that gonad shields were often poorly used, with inappropriate shield size and obscuration of important anatomy and pathology.14,27
Retrospective studies demonstrate a higher incidence of inaccurate gonadal shield placement in females compared to males (Figure 1).5,14,23-28 This was attributed to the varying position of the ovaries due to age and bladder volume, there is also lot of variability in the position of patients’ ovaries regardless of age or bladder volume.14,29-31 The ovaries descend from the posterior abdominal wall to their final position posterolateral to the uterus in the true pelvis which continues after birth into adolescence.32,33 A study using magnetic resonance imaging (MRI) to investigate the position of the ovaries for women between the ages of 16-59-years-old and children from birth to 15-years discovered that there is a significant association (p<0.0001) between young patients and their ovaries positioned outside of the true pelvis.31 Similarly, Bardo et al34 established that the ovaries were almost always positioned laterally in the pelvis for newborn to 18-year-old females. The effect of bladder emptying on the ovary position was dramatic, 65.2% of female children were found to have one or more ovaries outside of the ring of the pelvis when the bladder was full, whereas 27.8% shared the same result when the bladder was empty.31 The fluctuation of where the ovaries lie makes it difficult for radiographers to successfully protect them with gonad shields.
Figure 1. Inaccurate Gonad Shielding in Males Compared to Females from Review of the Literature
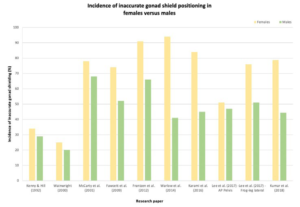
Tsai et al30 proposed improvements to gonad shield design to overcome issues of inappropriate selection of shield size and unpredictable location of the ovaries. They suggest using a pentagon-shaped gonad shield that is customised according to the distance between an imaginary line connecting the two anterior superior iliac spines (ASIS).35 ASISs are easily identifiable anatomical landmarks located close to the ovaries. The proposed gonad shield has its apex pointing toward the symphysis pubis with a plastic strip that extends 5 mm on each side which the radiographer uses to determine the patient’s ASIS and position the shield accordingly. Although the proposed shield may introduce artefacts from its plastic strip, the idea that gonad shields can be reformed to overcome the current dilemma can pose as motivation for future research.
Obscuring diagnostic information:
Inaccurate positioning of gonad shields hinders diagnostic information. Lines such as Shenton’s line, Perkin’s line, Klein’s line and the acetabular angles, which play important roles in confirming diagnoses such as developmental dysplasia of the hip (DDH), can be concealed by the gonad shield.23,36 Additionally, pathological appearances may be obscured by the shield and this was illustrated in Frantzen et al5study where the gonad shield covered a lucent lesion on the pubic rami. Ultimately, gonad shielding may eliminate useful diagnostic information when positioned incorrectly – an argument proposed by several articles promoting the discontinuation of gonadal shields.5,14,23,25
Dose implications:
Inaccurate positioning of gonad shields has dose implications for patients, which can negate the initial purpose of applying the gonad shields. One study performed Monte Carlo stimulations on anthropomorphic phantoms to compare how effective gonadal shields were when they were placed correctly, in comparison to when they were misplaced. It was found that when the gonad shield was misplaced by >6 cm in adults and >4 cm in 5-year-olds and newborns, dose reduction was less than 10% effective.37 Repeat X-rays may be required if errors in gonad shield positioning exist exposing the patient to increased radiation dose.14,22,25,28,38
The use of AEC is common in pelvic examinations and it terminates radiation exposure once a pre-set amount of radiation has been reached to produce an optimal optical density.2 However, a dense object will cause greater attenuation of the primary beam which will lead to longer exposure time, and subsequently increased dose to the patient, from the AEC.20 Therefore, when using AEC, it is important that patient positioning is accurate and the correct chambers are selected because this will result in either an unexposed or overexposed image. When a radio-opaque item such as a gonad shield is placed over an ionisation chamber, greater attenuation of the primary beam occurs and the AEC will increase the amount of radiation output in an attempt to penetrate through the lead in the gonad shield.20 In order to investigate the effects of using gonad shields with AEC, Kaplan et al29 and Davies et al20 measured the dose area product (DAP) to provide an indication of the dose and the amount of irradiated tissue.2 Kaplan et al29 demonstrated that when AEC chambers are used with gonad shields in female pelvic examinations, there is an increase in absorbed dose to unshielded and more radiosensitive organs, such as the colon and stomach due to the fact that inadequate photons are reaching the AEC as it is superimposed by the Pb. They recorded an increase of 63% in the 5-year-old phantom and 147% in the adult phantom in DAP, when AEC was used with gonad shields.29 Similarly, Davies et al20 demonstrated an increase in DAP by 23% when a gonad shield was applied to a female adult anthropomorphic phantom. On the contrary, another recent study performed on an anthropomorphic female adult argues that shielding with AEC or manual exposure does reduce the DAP and overall dose to the ovaries.39 However, this is only guaranteed in the clinical setting when the shield is positioned correctly, and no repeats are required. Furthermore, when a gonad shield was applied, unshielded organs had an increase in absorbed organ dose of 21-51% in the 5-yearold phantom and 17-100% in the adult phantom.29 As mentioned previously, younger patients tend to have their ovaries outside of the true pelvis or located laterally, therefore there is a potential increase in the absorbed dose to the ovaries when gonad shields are applied, which contradicts the original intent of applying one.31,34
Benefits of Lead Protection in Conventional Imaging
Reduce cumulative radiation dose:
Some diseases diagnosed at a young age require follow-up imaging and this places patients in a position where they will accumulate radiation dose over time. Two studies investigating pelvic X-rays in patients below the age of 16-years highlighted how frequently young patients had to undergo imaging.21,22 Sikand et al.21 found that 45% of their participants had more than one X-ray and some also had to have computed tomography (CT) scans and fluoroscopy. Two-years later, Gul et al22 identified 60% of their participants had more than 5 pelvic X-rays, with each patient having an average of 9 projections performed. Some medical conditions that may place young children in this situation include DDH, slipped upper femoral epiphysis (SUFE) or scoliosis. 1 in 1,000 children are likely to have DDH, with a high prevalence of 80% in females.38,40 Ultrasound is the preferred imaging modality for patients under 4-months old, but X-ray imaging is required as ossification begins in the femoral epiphyses. Even those children with suspected DDH who obtain a normal ultrasound are recommended to have follow-up X-rays until they start to walk for early diagnosis and intervention.41,42 Likewise, patients with SUFE are exposed to radiation in their initial anteriorposterior (AP) pelvis and frog-leg projections followed by images taken during surgery and routine follow-up X-rays.43
Adolescent idiopathic scoliosis (AIS) investigation and surveillance involves a full evaluation of the spine on the AP/ posterior-anterior (PA) and lateral views extending from the base of the skull to the pelvis.44,45 A study investigating the amount of ovarian radiation absorbed in young patients with scoliosis discovered that these patients received an average of 14 X-rays and 180 μSv cumulative ovarian dose per patient over 44-months.46 Presciutti et al47 investigated the doses associated with three types of treatments for AIS including posterior spinal fusion (operative), brace management (non-operative) and observation. They found that patients in the operative group received an average of 12 X-rays per year, 6 for the braced group and 3 for the observed group. Furthermore, patients undergoing surgery required prior radiographs, imaging during surgical procedure and post-operative CT scans.
Hence, they concluded that patients in this group received the greatest average annual radiation dose. Another issue with imaging of patients with AIS is that repeated radiographs are common. Oetgen et al45 found that when patients were referred to a specialist, 43% of patients required repeated imaging due to inadequate radiographs as a result of exclusion of anatomy, missing views and inability to measure the angle, not incorrect placement of lead. Thus, for patients with AIS, gonad shielding could be beneficial in the long-term.
Moreover, some patients, as young as neonates, are monitored with daily chest X-rays because of severe illnesses. Testicular entrance skin dose (ESD) was measured from a neonatal phantom and they found that by applying a gonad shield, 59% reduction in testicular ESD can be achieved.48 They measured the ESD without gonadal shielding to be 3×10-4 mGy, with lead shielding to be 1.4×10-4 mGy and the cumulative dose acquired over a period of 31-days is approximated to be 0.0105 mGy.48 Although this study was performed in 1997, a more recent study in 2020 proposed there may be a possible correlation between diagnostic exposure and the probability of getting testicular germ cell tumour (TGCT). The study found that there was a statistically significant increase (p=0.01) risk of TGCT for patients with more than 3 exposures in X-ray or CT, compared to no exposure.49,50
LIMITATIONS
Many retrospective studies were included in this literature review.5,14,22,24,26,27,30,47 Retrospective studies can be unreliable and are subject to numerous issues with reliability and limit the interpretation and generalizability of the results.51 Karami et al27 acknowledge that the rejected images that may have led to the repeat were not included in the study due to the retrospective nature of image retrieval from picture archiving and communication system (PACS). As such it cannot be established if it was actually due to poor positioning of the lead that led to the repeat image. In other cases, it appears that the results relating to why repeat imaging was performed was speculative based on the fact that a repeat was performed rather than an actual review of the image.5,14,27 Franzen et al5 state that due to an important landmark being obscured by the lead that a repeat image was required in 28% of images on females and 4.14% of males. In the footnote they state that a repeat was not actually required which would suggest that the image was in fact diagnostically acceptable.
Some studies recorded the number of plain radiographs pediatric patients in their sample size had over a period but other modalities involving ionizing radiation were excluded from the study.22,46 This information would be useful in determining cumulative dose of the patient over time, which is an important factor in estimating radiation risk.
Furthermore, as some observers were given a grading criterion while assessing radiographic images, optimal inter-observer reliability was not achieved as interpretation of the criteria remained subjective.20 The fact that the quantity of repeated images could not be obtained could also mean the recorded radiation exposures were likely underestimated.47
One size anthromorphic phantom was used in many research papers which does not reflect the variation in patients undergoing medical imaging examinations.20,29,37,46,48 Only one study investigating the effects of applying gonad shields in conjunction with AEC accounted for the difference in varying body sizes.39 Studies should include a range of phantom sizes to replicate a clinical setting.
Articles that investigated the effect of gonad shields on the dose received by patients utilise the DAP. However, the DAP has uncertainties (±5-10%) associated with them from the dose meters even when they are calibrated.5,20 The uncertainty is increased when correction is applied for shuttering to obtain the true X-ray field.5 Furthermore, the Monte-Carlo program, program for X ray monte carlo (PCXMC), used in Frantzen et al5 and Davies et al20 studies to calculate patients’ effective and organ doses poses some limitations. Firstly, the size of the phantom may not correspond to the size of the patient at the age because there was only a limited number of phantoms to calculate from within the program.5 Secondly, having a gonad shield within the collimation field may have decreased the accuracy of the results because it is not accounted for in the program.20
FUTURE CONSIDERATIONS
As practice evolves, it is imperative to establish standardised protocols for the use of gonad shields to ensure consistency in practice that are firmly supported by the best available research. Research papers recognise the underlying issue of using gonad shields in patients, and most of them recommend reconsidering their effectiveness in female patients due to the unpredictable location of the ovaries. It is possible that Pb shielding might be omitted for female patients but still utilised for male patients, but this raises ethical dilemmas where one group is protected and another group is not. Many professional and governing bodies support the abandonment of Pb gonadal shielding. In Australia both the Australian Society for Medical Imaging and Radiation Therapy (ASMIRT) and the Australasian College of Physical Scientists and Engineers on Medicine (ACPSEM) have endorsed the AAPM recommendation to abandon the use of lead protection for patients. However, it should be noted that the Australian Radiation Protection and Nuclear Safety Agency (ARPANSA), the Australian Government’s primary authority on radiation protection and nuclear safety, has not yet published updated guidance on the use of patient shielding.
More research exploring novel gonad shielding designs for females that account for unpredictable location of the ovaries could help to overcome positioning errors in this population.
Monitoring of the cumulative dose that a member of the public obtains over their lifetime from medical imaging examinations or for a paediatric sample who requires follow-up imaging due to an underlying condition is very challenging and so radiographers must justify every radiographic exposure to ensure that the benefits outweigh any risks.
Other methods effectively reduce radiation dose to patients, and radiographers must not lose sight of these as the conversation shifts to lead protection. Any conversation about reduced dose to patients must be in conjunction with proper collimation, filtration, justification of the examination before going ahead and continuous enhancement of radiographic technique to prevent repeated images.14
Many papers suggest that the amount of radiation reduction achieved by lead shields is negligible, but not zero. In according to the as low as reasonably achievable (ALARA) principle that is embedded into radiographic practice, radiographers must strive toward obtaining the lowest possible radiation dose, which should include any benefits even negligible ones. Although the tissue weighting factor of gonads does not compare with other radiosensitive tissues such as breast and colon, this alone should not justify neglecting safe radiation practice.
CONCLUSION
In a highly scientific field of work, variations in practice of gonadal protection during X-rays may cause misconceptions for patients.
There is a high incidence of inaccurately positioned gonad shields for female patients, which leads to repeated X-ray images. Using gonad shields with AEC chambers increased dose to patients. The use of gonadal shielding however is beneficial for patients requiring frequent X-ray examinations as it can reduce cumulative radiation dose. Due to the longer life expectancy of younger patients, the effects of radiation can be observed in the future. Radiographers should be actively involved in reducing unnecessary or avoidable exposure to patients. Therefore, radiographers need to understand when gonadal shielding offers protection to patients and be skilled in its use. In Australia at least until such time as ARPANSA updates its radiation safety guides, a gap seems to exist with regard to abandoning lead protection at this time. Establishing a standardised protocol regarding the application of gonadal shields, supported by regulation agencies, is imperative. Varying practice amongst radiographers can cause unnecessary concerns from patients, which must be avoided.
ETHICAL CONSIDERATIONS
This study did not require ethical review board consideration as it was a review of the literature.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.






