CASE REPORT
A 37-year-old multiparous woman presented during routine ward follow-up with a headache day two following a declared uncomplicated lumbar epidural for labour analgesia.
Past medical history included essential hypertension, controlled with methyldopa. A previous pregnancy was complicated by pre-eclampsia and a post dural puncture headache with recognised dural tap. This had been managed conservatively, with no further follow-up. Of note, there is no previous history of chronic headache prior to pregnancy.
On this occasion there was no dural tap noted at the time of insertion. Two epidural attempts with a 16-gauge Tuohy needle were undertaken at the L3-L4 interspace before a successful attempt at a lower lumbar level. The epidural behaved appropriately following a test, main dose and patient controlled analgesia infusion.
Day two after an uneventful vaginal delivery the patient was routinely seen on the ward and noted to have a postural headache of moderate severity. There were no other concerning features in the history at this time and neurological examination was normal. A working diagnosis of post dural puncture headache was made, and the patient managed conservatively with follow-up arranged for the following day.
On the third day postpartum, the headache had increased to an analogue score of severe accompanied by neck rigidity and photophobia. The patient reported right arm pain and subjective weakness. Neurological examination, including objective assessment of limb power, remained normal.
Non-contrast magnetic resonance imaging (MRI) head and spine imaging was arranged urgently for the same day looking for the intracranial correlates of and possible causes of the working diagnosis – dural breach with consequent intracranial hypotension. Bilateral cerebral hemispheric shallow hygromas were seen on the flair and T1 sequences, with descent of the aqueduct but no tonsillar descent (Figure 1). The brain parenchyma was otherwise normal. Extra axial hygroma collections were also seen from the foramen magnum inferiorly to the level of C6 (Figure 2) with sagittal cuts suggesting that the collections extended into the lumbar region.
Figure 1. MRI brain a) Coronal FLAIR and b) axial T2 Weighted Image Demonstrate Bilateral Hygromas (white arrows)
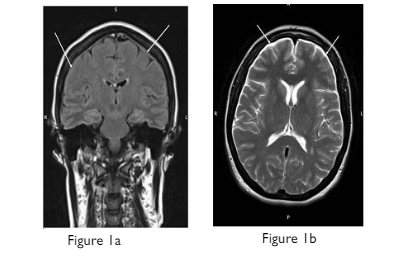
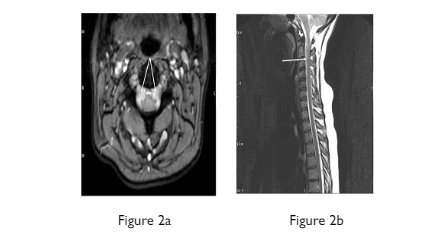
Figure 2. MRI Cervicothoracic Spine a) axial T2 and b) sagittal T2 Reveal Subdural CSF Collections (white arrows) Extending Inferiorly into the
Vertebral Canal with Slight Displacement of the spinal cord (arachnoid dissection)
A neurosurgical opinion advised supine bed rest overnight and observation. If no improvement in symptoms followed, an epidural blood patch should be considered once any source of infection had been discounted.
In the absence of improvement of symptoms an epidural blood patch with 24ml of autologous blood was performed electively on day four without event. This was delivered at the level of L3-4 and the patient was managed in bed for two hours afterwards. The following day the patient, having been symptom free for over twelve hours, mobilised with ease and was discharged.
At four-week follow-up, with repeat MR imaging, the patient remained asymptomatic and the MRI showed complete resolution of the previously seen changes.
DISCUSSION
Subdural hygroma formation following epidural analgesia in labour is not widely reported in the literature. The true incidence is unknown but thought to be rare.1 There is a possibility that such cases, if asymptomatic, may go largely undetected. There is no data on persistence of low-pressure symptoms in this setting. This case report describes the more unusual finding of both intracranial and spinal subdural collections following a lumbar procedure.
Subdural hygromas are collections of interstitial and xanthochromic fluid that can develop within the subdural space following trauma to the dura.2,3 This is seen more commonly following head injury and also as a complication following neurosurgery or spinal anaesthesia.4,5,6 Following dural puncture, cerebrospinal (CSF) leakage into the epidural spinal space can lead to intracranial hypotension and a resultant vasodilation of adjacent vessels. Effusion of interstitial fluid across a pressure gradient, from these dilated vessels, is thought to be the mechanism for hygroma formation intracranially. Intracranial subdural hygromas can be unilateral or bilateral, and rarely extend caudally.2,3,4 Spinal CSF collections in the intradural, extra-arachnoid space are otherwise identified as spinal arachnoid dissections. This complication following a spinal or epidural anaesthesic is very rare.7,8 Collections usually occur secondary to lumbar spine surgery, with, although not exclusively so, durotomy. These dissections can be close or distant from the surgical site. It is likely, though unproven, that the exact mechanism of such collections within the cranium and the spine is different.9,10,11
Subdural hygroma can present clinically with postural headache, neck stiffness, photophobia, nausea or vomiting, focal neurological deficits, seizures and even reduced level of consciousness. The total volume of extra-arachnoid CSF is likely to contribute to the extent of the symptoms.4 When extra-axial collections form, peripheral neurology could also be compromised.9 The site of the spinal hygroma may not by correspond to the initial dural tear.
Subdural hygroma after unrecognised dural puncture is likely to be under diagnosed. MRI is the investigative modality of choice, as these low-density fluid collections may be difficult to discriminate from haematoma on computed tomography (CT). Diagnosis is important as hygromas can progress to subdural haematomas. The mechanism is suggested to be secondary to traction on, and tearing of, venous blood vessels. Following the well recognized subdural hygroma formation secondary to traumatic brain injury, progression to subdural haematoma is seen in 8.2% of patients.3 Significant intracranial hypotension with ongoing precipitant CSF leak may progress to cerebellar herniation in untreated severe cases.
Management of uncomplicated subdural hygromas is focused on conservative methods whilst awaiting the natural history of spontaneous leak resolution. Conservative methods may include supine rest, hydration, caffeine and analgesia.4 Symptomatic relief from low-pressure symptoms, without hygroma collection, can also be delivered with an occipital nerve block, if required.12,13 The effused fluid will reabsorb and be redistributed.2 In patients who remain symptomatic, an epidural blood patch may be considered. This is thought to either repair the dural breech or displace a comparable volume of CSF therefore treating the hypotension whilst the natural history occurs.4
Neurosurgical intervention is extremely uncommon but should be considered in complicated or non-resolving cases. Any progression in symptoms warrants further neurological imaging to assess for advancing intracranial pathology.2,7
The risk of occult dural breech makes diagnosis of post dural puncture headache more difficult.14 This case demonstrates the importance of structured anaesthetic follow-up after epidural labour analgesia, and the need to maintain high index of suspicion for pathological sequelae in atypical headaches or neurology with a low threshold for imaging and investigation.







