INTRODUCTION
The coronavirus disease-2019 (COVID-19) pandemic has disrupted modern human living since its onset. As of 22 September 2022, there were about 610 million reported cases worldwide with near to 6.5 million deaths.1 Certain population groups are at higher risk of severe infection and poor outcomes, including patients with autoimmune rheumatic diseases (ARD).2
MATERIALS AND METHODS
This is a retrospective observational study involving COVID-19 infections among the patients with ARD encountered at our rheumatology unit, in the state of Kelantan. The cases were identified through our local healthcare network and patients’ own narration, from March 2020 until April 2022. The data were analyzed with both parametric and non-parametric tests using StatPlus, which included Kruskal-Wallis analysis of variance (ANOVA) and logistic regression. The infection severity was classified based on the National Institute of Health (NIH) (US) guidelines.2 Whereas the infection outcomes comprised 3 components: full recovery, sequelae and fatality.
RESULTS
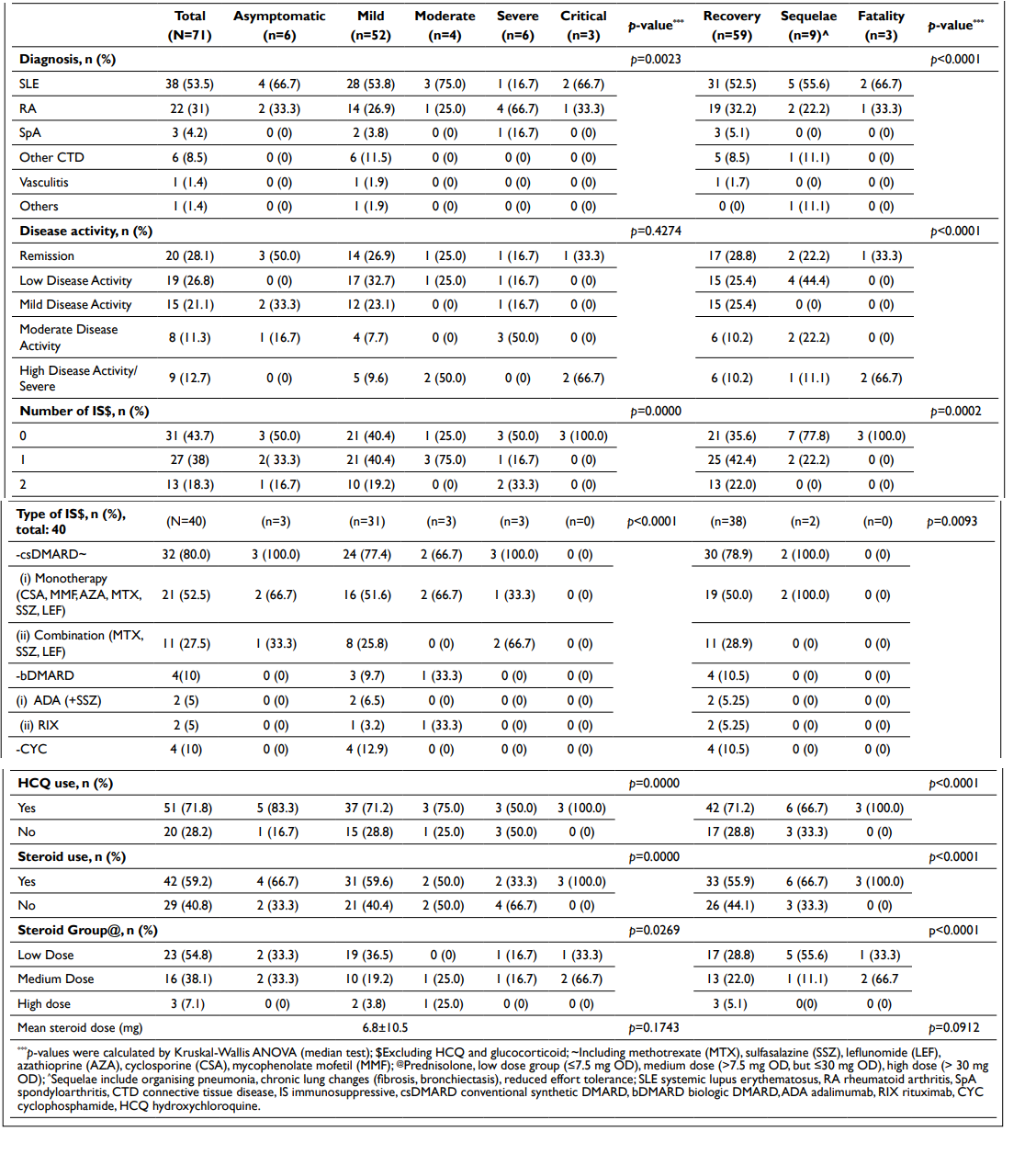
A total of 71 confirmed cases of COVID-19 infection were recorded, affecting 62 patients. Nine (9) patients had recurrent infections. Fifty-nine point two percent (59.2%) of infections occurred during the Delta variant dominance period, followed by 36.6% within the Omicron variant dominance period. Ninety-five point eight percent (95.8%) of cases involved female patients. All were of South East Asian descent with a median age of 39-yearsold and 66.2% were less than 50-years-old.
The most common ARD diagnosis was systemic lupus erythematosus (SLE) (53.5%), followed by rheumatoid arthritis (RA) (31%). Most patients (54.9%) attained low disease activity and remission prior to infection. 56.3% (40/71) had received at least one IS [excluding hydroxychloroquine (HCQ) and steroids], mostly conventional synthetic disease-modifying antirheumatic drugs (DMARDs) (80%)—methotrexate (mono-/combination therapy)(19) the commonest, followed by leflunomide (mono-/ combination therapy) (9) and azathioprine (7). Mycophenolate mofetil use was seen in 3 patients.
Ten percent (10%) (4/40) of patients were prescribed biologic DMARDs [tumour necrosis factor-α (TNF-α) inhibitor and rituximab] and 10% (4/40) had ongoing IV cyclophosphamide therapy. Seventy-one point eight percent (71.8%) of patients were on HCQ prior to infection too. As many as 59.2% of patients received steroids at the mean dose of 6.8±10.5 mg OD. Forty-five point two percent (45.2%) consumed medium-to-high doses of prednisolone.
Regarding COVID-19 vaccination, 71.9% of patients received at least 1 dose of the vaccine. Messenger ribonucleic acid (mRNA) monovalent vaccine (Pfizer-BioNTech, NY, USA) was the commonest COVID-19 vaccine (76.4%) prescribed, followed by inactivated COVID-19 vaccine (CoronoVac) (15.7%). Two percent (2%) received a heterogeneous combination of mRNA and inactivated COVID-19 vaccines (primary series of CoronoVacCoronoVac-Pfizer BT, NY, USA). Only 57.7% of patients had complete vaccination (≥2 doses) and a mean of 4.2±1.8-months elapsed after the last vaccine dose prior to the infection in this patient group.
We had identified the risk factors for severe COVID-19 as recommended by the Centers for Disease Control and Prevention (CDC), US, and COVID-19 Global Rheumatology Alliance (GRA) registry.3 Seventy-seven point five percent (77.5%) of our patients had at least 1 risk factor portending severe COVID-19 infection. As many as 61.9% were either overweight or obese. The other common comorbidities identified were hypertension (22.5%), chronic lung diseases (22.5%) (which included bronchial asthma and connective tissue disease-related interstitial lung diseases, CTD-ILD), and diabetes mellitus (12.7%). One (1) patient (1.4%) had concomitant connective tissue disease-related interstitial lung disease (CTD-ILD) and pulmonary hypertension. None of these patients were smokers.
Of note, 5 COVID-19 infection cases occurred during pregnancy, involving 4 SLE patients. Three (3) had asymptomatic/mild infection with underlying SLE in mild disease activity (1/3) or remission (2/3). Prednisolone 10 mg once daily was prescribed to the patient with mild disease activity. All were on HCQ but did not have a steroid-sparing agent. One (1) pregnancy patient had recurrent infections and experienced critical illness at the second infection, for which she received IV tocilizumab therapy. It was complicated by organizing pneumonia. She had high disease activity prior to both incidents and was on steroid therapy (Table 1). No obstetric complications were documented in all cases, nevertheless.
Table 1. Patients with Recurrent COVID-19 Infections
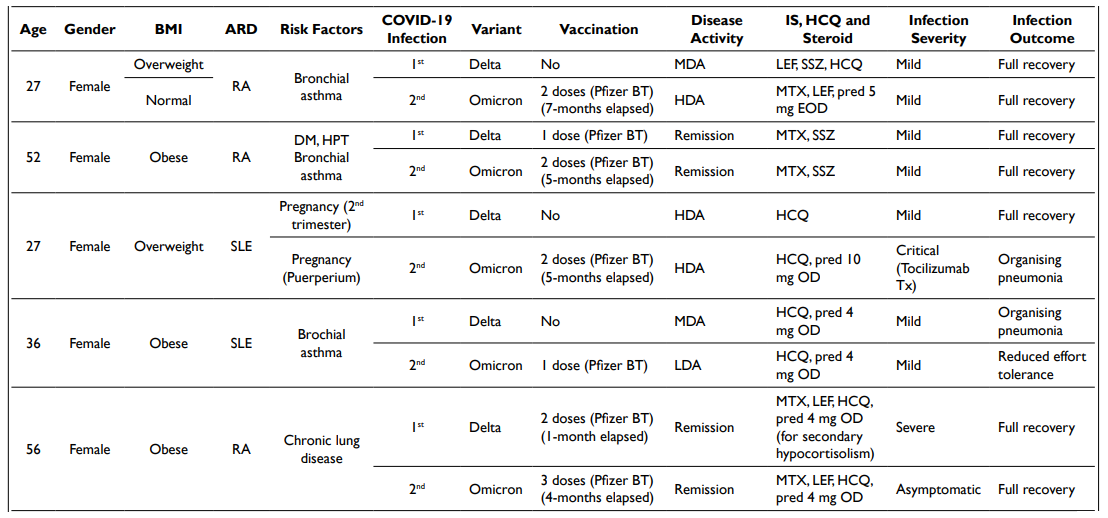
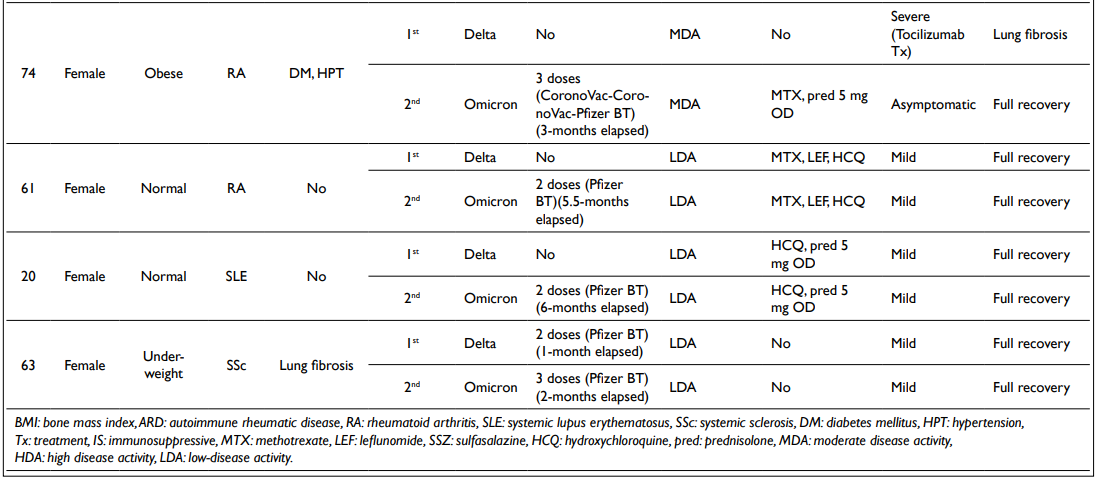
A total of 9 patients had experienced recurrent infections (Table 1). All but one had complete vaccination prior to the second infection. Seventy-seven point eight percent (77.8%) had risk factors for severe infection, with overweight and obesity being the majority (66.7%), followed by chronic lung diseases (55.6%). In 38.9% of cases, the patients had moderate to high disease activity. Overall, most infections were asymptomatic to mild (83.3%) and 77.8% of the patients recovered fully.
Likewise in the overall study population, most of the COVID-19 infections were asymptomatic and mild (81.7%). Fourteen point one percent (14.1%) had moderate to severe infection and 4.2% were in the critical stage as defined by the NIH.2 A total of 3 deaths were recorded, rendering case fatality rate 4.2%. These patients were relatively young females, one was fully vaccinated and two were, however, unvaccinated (Table 2).
| Table 2. COVID-19 Case Fatality |
| Age |
Gender |
BMI |
ARD |
Risk Factors |
Variance Dominance |
Vaccination |
Disease Activity |
IS and Steroid |
Infection Severity |
Remark |
| 46 |
Female |
Normal |
SLE |
HPT |
Delta |
2 doses (2-months elapsed) |
HDA (Active LN) |
HCQ, pred 30 mg OD |
Critical |
Death at stage 1 of COVID-19 infection |
| 23 |
Female |
Normal |
SLE |
No |
Omicron |
No |
HDA (Active LN & probable lupus mesenteric vasculitis) |
HCQ, pred 10 mg OD |
Moderate |
Death at stage 1 (Possible cause of death: acute abdomen – no post-mortem) |
| 51 |
Female |
Obese |
RA |
HPT, DM with infection proneness (recurrent nasofacial abscess, H. pylori infection) |
Delta |
No |
Remission |
HCQ, pred 5 mg OD (for secondary hypocortisolism) |
Critical |
Death at stage 3 Was initially admitted for CMV esophagitis (on IV antiviral therapy) |
| BMI: bone mass index, ARD: autoimmune rheumatic disease, RA: rheumatoid arthritis, SLE: systemic lupus erythematosus, LN: lupus nephritis, DM: diabetes mellitus, HPT: hypertension, IS: immunosuppressive, HCQ: hydroxychloroquine, pred: prednisolone, HDA: high disease activity |
Subsequent statistical analyses (Kruskal-Wallis ANOVA) revealed significant differences between stratified demographic and clinical characteristics in terms of severity associated with COVID-19 infection (Tables 3 and 4). A similar observation was found in relation to the outcomes of the infection too.
Table 3. Demographic Data, COVID-19 Infection Severity and Outcome
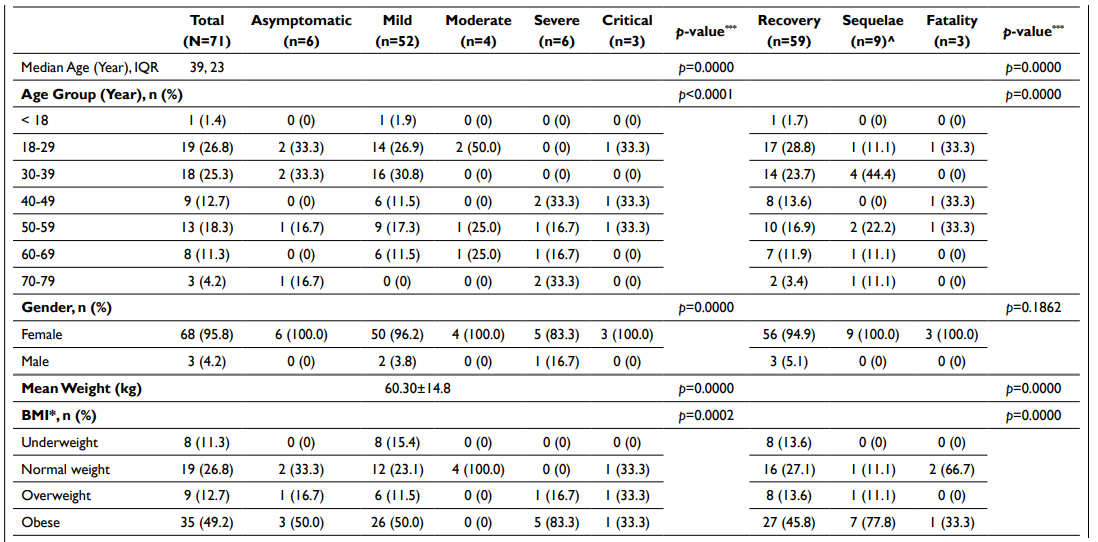
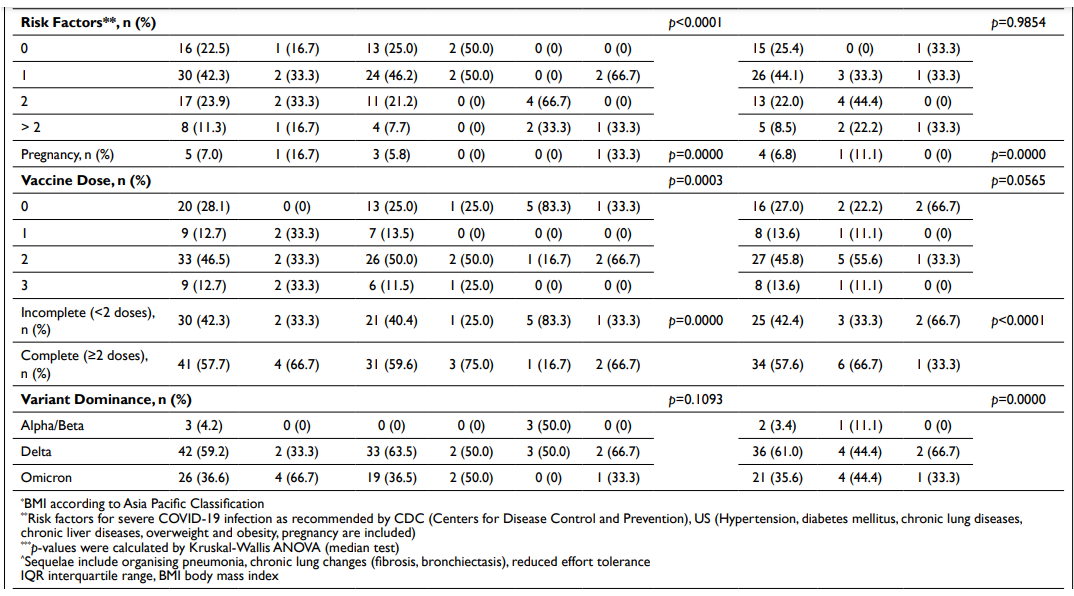
Table 4. Clinical Entities, COVID-19 Infection Severity and Outcome
We performed logistic regression (LR) on various confounding factors as listed in Table 5. From the univariate LR analyses, we found that severe COVID-19 infection could be predicted by the number of vaccines received [OR -0.22 (-0.42, -0.02), p=0.03], ARD activity [OR 0.16 (0.01, 0.32), p=0.04], and COVID-19 variant type [OR -0.52 (-0.88, -0.15), p=0.006]. The use of IS could be associated with better infection outcomes [OR -0.37 (-0.60, -0.14), p=0.002] and reduced fatality [OR 0.097 (0.002, 0.191), p=0.05]. The number of IS used might seem to affect the infection outcomes too [OR -0.23 (-0.38, -0.08), p=0.003]. Interestingly, patients who had biologic DMARDs (TNF-α inhibitor and rituximab), as well as IV cyclophosphamide therapy, might fare better than those on conventional synthetic DMARDs in terms of infection outcomes [OR -0.11 (-0.19, -0.02), p=0.01].
| Table 5. Logistic Regression Analyses of Factors Associated with COVID-19 Severity, Outcome and Fatality |
|
Univariate |
Multivariate |
| Severity |
Outcome (O)* & Fatality (F) |
Severity |
Outcome (O)* & Fatality (F) |
| All Cases (N=71) |
OR (95% CI), p-value |
OR (95% CI), p-value |
OR (95% CI), p-value |
OR (95% CI), p-values |
| Age |
0.01 (-0.0004, 0.0274), p=0.06 |
O: 0.002 (-0.01, 0.01), p=0.70 |
0.01 (-0.08, 0.10), p=0.83 |
O: -0.01 (-0.06, 0.04), p=0.80 |
| F: 0.0003 (-0.003, 0.004), p=0.86 |
F: -0.003 (-0.02, 0.02), p=0.81 |
| Age group |
0.13 (-0.01, 0.26), p=0.06 |
O: 0.02 (-0.06, 0.1), p=0.60 |
0.06 (-0.72, 0.83), p=0.89 |
O: 0.10 (-0.36, 0.56), p=0.66 |
| F: 0.0007 (-0.03, 0.03), p=0.97 |
F: 0.003 (-0.19, 0.20), p=0.97 |
| Gender |
0.42 (-0.64, 1.47), p=0.43 |
O: -0.22 (-0.82, 0.38), p=0.46 |
-0.12 (-1.33, 1.08), p=0.84 |
O: -0.43 (-1.14, 0.29), p=0.24 |
| F: 0.04 (-0.20, 0.28), p=0.71 |
F: 0.10 (-0.21, 0.40), p=0.52 |
| Weight |
0.00 (-0.01, 0.02), p=0.79 |
O: 0.0009 (-0.007, 0.009), p=0.83 |
0.002 (-0.03, 0.03), p=0.89 |
O: -0.002 (-0.02, 0.01), p=0.83 |
| F: 0.0005 (-0.003, 0.004), p=0.78 |
F: -0.003 (-0.01, 0.005), p=0.49 |
| BMI |
0.05 (-0.14, 0.25), p=0.55 |
O: 0.05 (-0.06, 0.16), p=0.85 |
0.04 (-0.35, 0.43), p=0.83 |
O: 0.10 (-0.13, 0.33), p=0.41 |
| F: 0.01 (-0.03, 0.06), p=0.60 |
F: 0.02 (-0.08, 0.12), p=0.63 |
| Vaccine dose |
-0.22 (-0.42, -0.02), p=0.03* |
O: -0.05 (-0.16, 0.07), p=0.42 |
-0.43 (-0.93, 0.06), p=0.09 |
O: -0.14 (-0.43, 0.15), p=0.34 |
| F: 0.03 (-0.02, 0.08), p=0.19 |
F: 0.06 (-0.07, 0.18), p=0.37 |
| Complete vaccination |
-0.23 (-0.66, 0.20), p=0.29 |
O: -0.04 (-0.28, 0.21), p=0.76 |
0.87 (-0.08, 1.81), p=0.07 |
0.87 (-0.08, 1.81), p=0.07 |
| F: 0.04 (-0.06, 0.14), p=0.39 |
F: -0.03 (-0.27, 0.21), p=0.77 |
| ARD Dx |
-0.15 (-0.52, 0.22), p=0.42 |
O: 0.03 (-0.19, 0.24), p=0.81 |
0.21 (-0.31, 0.73), p=0.42 |
O: 0.01 (-0.30, 0.32), p=0.97 |
| F: 0.01 (-0.07, 0.10), p=0.77 |
F: 0.02 (-0.11, 0.15), p=0.75 |
| Risk Factors (number) |
0.19 (-0.004, 0.38), p=0.05 |
O: 0.07 (-0.04, 0.18), p=0.22 |
0.13 (-0.17, 0.43), p=0.39 |
O: 0.02 (-0.16, 0.20), p=0.81 |
| F: -0.001 (-0.05, 0.04), p=0.95 |
F: 0.01 (-0.07, 0.09), p=0.81 |
| Disease activity |
0.16 (0.01, 0.32), p=0.04* |
O: 0.05 (-0.03, 0.14), p=0.22 |
0.16 (-0.05, 0.36), p=0.13 |
O: 0.07 (-0.06, 0.19), p=0.30 |
| F: -0.03 (-0.06, 0.01), p=0.14 |
F: -0.02 (-0.07, 0.04), p=0.54 |
| IS use |
-0.27 (-0.69, 0.16), p=0.21 |
O: -0.37 (-0.60, 0.14), p=0.002* |
-0.49 (-1.59, 0.61), p=0.37 |
O: -0.28 (-0.93, 0.37), p=0.39 |
| F: 0.097 (0.002, 0.19), p=0.05* |
F: 0.06 (-0.22, 0.34), p=0.66 |
| Number of IS |
-0.13 (-0.42, 0.15), p=0.36 |
O: -0.23 (-0.38, -0.08), p=0.003* |
0.32 (-0.47, 1.11), p=0.42 |
O: -0.04 (-0.51, 0.43), p=0.86 |
| F: 0.06 (-0.01, 0.12), p=0.08 |
F: 0.01 (-0.20, 0.21), p=0.95 |
| Type of IS |
0.07 (-0.22, 0.09), p=0.41 |
O: -0.11 (-0.19, -0.02), p=0.01* |
-0.05 (-0.30, 0.21), p=0.70 |
O: -0.01 (-0.16, 0.14), p=0.90 |
| F: 0.02 (-0.01, 0.06), p=0.16 |
F: 0.005 (-0.06, 0.07), p=0.88 |
| HCQ use |
0.05 (-0.51, 0.43), p=0.85 |
O: 0.09 (-0.18, 0.35), p=0.53 |
0.12 (-0.46, 0.69), p=0.70 |
O: -0.05 (-0.39, 0.30), p=0.78 |
| F: -0.06 (-0.17, 0.05), p=0.27 |
F: -0.04 (-0.18, 0.11), p=0.57 |
| Steroid use |
-0.01 (-0.45, 0.42), p=0.95 |
O: 0.18 (-0.06, 0.42), p=0.14 |
-0.01 (-1.11, 1.08), p=0.98 |
O: 0.39 (-0.25, 1.03), p=0.23 |
| F: -0.07 (-0.17, 0.03), p=0.15 |
F: -0.12 (-0.40, 0.16), p=0.39 |
| Steroid group |
0.05 (-0.19, 0.29), p=0.67 |
O: 0.06 (-0.07, 0.20), p=0.36 |
-0.08 (-1.00, 0.83), p=0.85 |
O: -0.19 (-0.74, 0.35), p=0.47 |
| F: -0.04 (-0.09, 0.01), p=0.13 |
F: 0.06 (-0.17, 0.30), p=0.59 |
| Steroid dose |
0.01 (-0.01, 0.03), p=0.25 |
O: 0.003 (-0.01, 0.1), p=0.58 |
0.02 (-0.02, 0.07), p=0.34 |
O: 0.01 (-0.02, 0.03), p=0.60 |
| F: -0.003 (-0.01, 0.01), p=0.17 |
F: -0.005 (-0.02, 0.01), p=0.42 |
| Variant dominance |
-0.52 (-0.88, -0.15), p=0.006* |
O: 0.01 (-0.21, 0.23), p=0.95 |
0.40 (-0.94, 0.15), p=0.15 |
O: 0.06 (-0.26, 0.38), p=0.70 |
| F: -0.001 (-0.09, 0.09), p=0.98 |
F: -0.04 (-0.18, 0.09), p=0.54 |
| Severity: R2=0.33, F(18, 70)=1.41, p=0.16; Outcome: R2=0.27, F(18,
70)=1.05, p=0.42
Fatality: R2=0.16, F(18, 70)=0.56, p=0.91 |
| Subgroup analyses (Patients with complete vaccination, N=41) |
| Duration from last vaccine dose |
-0.09 (-0.23, 0.05), p=0.21 |
O: -0.03 (-0.11, 0.05), p=0.42 |
-0.005 (-0.23, 0.22), p=0.97 |
|
| F: 0.02 (-0.01, 0.04), p=0.24 |
|
| Disease activity |
0.20 (0.01, 0.39), p=0.04* |
O: 0.06 (-0.04, 0.17), p=0.24 |
0.39 (0.009, 0.76), p=0.045 |
|
| F: -0.04 (-0.07, 0.001), p=0.04* |
|
| IS use |
-0.13 (-0.68, 0.42), p=0.63 |
O: -0.30 (-0.58, -0.01), p=0.04* |
-0.88 (-2.38, 0.61), p=0.23 |
|
| F: 0.06 (-0.04, 0.16), p=0.22 |
|
| Number of IS |
-0.07 (-0.44, 0.30), p=0.71 |
O: -0.20 (-0.40, -0.01), p=0.04* |
1.25 (0.06, 2.45), p=0.04 |
|
| F: 0.04 (-0.03, 0.11), p=0.28 |
|
| Steroid dose |
0.02 (-0.002, 0.045), p=0.07 |
O: 0.006 (-0.007, 0.199), p=0.34 |
0.002 (-0.08, 0.08), p=0.95 |
|
| F: -0.005 (-0.009, -0.0004), p=0.032* |
|
| Severity: R2=0.49, F(18, 40)=1.15, p=0.37; Outcome: R2=0.56, F(18,
40)=1.54, p=0.17
Fatality: R2=0.54, F(18, 40)=1.42, p=0.21
#Residuals Plot/Test Error – MSE=0 or d.f.=0 (in all analyses) |
| Subgroup analyses (Patients on IS, N=40) |
| Number of IS |
0.12 (-0.34, 0.58), p=060 |
O: -0.07 (-0.22, 0.08), p=0.33 |
0.46 (-0.37, 1.29), p=0.26 |
O: -0.06 (-0.40, 0.28), p=0.73 |
| Type of IS |
0.007 (-0.16, 0.17), p=0.94 |
O: -0.03 (-0.08, 0.03), p=0.31 |
-0.09 (-0.35, 0.18), p=0.50 |
O: -0.04 (-0.15, 0.07), p=0.49 |
#Fatality was not analysed due to absent variable (no fatal cases)
Severity: R2=0.52, F(17, 39)=1.38, p=0.24; Outcome: R2=0.25, F(17, 39)=0.44, p=0.96 #Fatality was not analysed due to absent dependent variable (no fatal cases) |
| Subgroup analyses (Patients on IS and with complete vaccination, N=25) |
| Number of IS |
0.03 (-0.53, 0.60), p=0.91 |
O: -0.11 (-0.37, 0.15), p=0.38 |
1.26 (-1.39, 3.91), p=0.30 |
O: -0.28 (-1.68, 1.13), p=0.66 |
| Type of IS |
0.02 (-0.16, 0.20), p=0.84 |
O: -0.04 (-0.12, 0.04), p=0.29 |
-0.63 (-2.69, 1.42), p=0.73 |
O: -0.01 (-1.10, 1.07), p=0.98 |
| #Fatality was not analysed due to absent variable (no fatal cases)
Severity: R2=0.50, F(17, 24)=0.42, p=0.93; Outcome: R2=0.34, F(17, 24)=0.21, p=0.996 #Fatality was not analysed due to absent dependent variable (no fatal cases) #Residuals Plot/Test Error – MSE=0 or d.f.=0 (in all analyses) |
| Outcome analysis included recovery, sequelae and fatality; BMI: body mass index, ARD: autoimmune rheumatic diseases, Dx: diagnosis, IS: immunosuppressive, HCQ: hydroxychloroquine |
We also performed analyses on patients with complete vaccination (≥2 doses) and found that active disease could predict severe infection [OR 0.20 (0.01, 0.39), p=0.04] and fatality [OR -0.04 (-0.07, 0.001), p=0.04]. The risk of fatality could be higher in patients who had high-dose steroids [OR -0.005 (-0.009, -0.0004), p=0.032]. In a similar manner, better infection outcomes could be associated with IS use [OR: -0.30 (-0.58, -0.01), p=0.04] and number of IS [OR -0.20 (-0.40, -0.01), p=0.04].
In subsequent multivariate LR analyses, however, we did not identify any predictive factors associated with severe infection, poor infection outcomes, and fatality associated with COVID-19 infection.
DISCUSSION
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the SARS-CoV-2 virus, which is genetically related to SARS-CoV and MERS-CoV. From the initial beta and alpha variants, now emerges a number of variants of concern and subvariants attributable to its rapid genomic mutations.
Since its escalation into the pandemic phase, experts and researchers in medical fields worldwide have been working hard on various effective preventive and therapeutic measures for COVID-19 infection. This armamentarium has seen advances in vaccine development enabling mass COVID-19-specific vaccine production within 1-year. Pfizer-BioNTech vaccine (an mRNA vaccine) was the first to receive emergency use authorization from the Food and Drug Administration (FDA) on 11th December 2020.4 A great number of therapeutic options are currently available, targeting at different facets of the infection (from direct viral inhibition and neutralizing monoclonal antibodies against viral spike protein to immunomodulation of enhanced host immune dysfunction).
Globally the rheumatology fraternity had kickstarted the COVID-19 Global Rheumatology Alliance (GRA) registry, engaging both the researchers and patient support or partner groups, with the effort to collect, analyze, and disseminate the data pertinent to COVID-19 infection in patients with rheumatologic diseases.3 Over the past 2-years, >20,000 patient records were registered and nearly 30 papers/articles were published. The available data has provided useful insights to inform rheumatologists worldwide and practical clinical guidelines from American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) had been produced.5,6,7,8,9,10
Patients with ARD, in general, are at increased risk of hospitalization, worse outcome, and death related to COVID-19 infection.11,12 This could be attributable to the confounding effect of ARD and the treatment regimen, and the burden of concomitant comorbidities and socio-epidemiological factors.13 Our study showed that most infections were mild/asymptomatic (81.7%), whereas severe and critical cases were 12.7%. Statistically, we could not report hospitalization as a key outcome of interest because our national policy had a lower threshold of hospitalization to both specialist and non-specialist facilities regardless of the severity of infection, posing a potential bias error. The case fatality rate was higher (4.2%) than those reported in both overall Malaysia (0.8%) and home state (Kelantan) records (0.6%).14 This high percentage, however, could partially be contributed by potentially lower denominator reported cases due to restricted sampling methods. Nevertheless, a higher mortality rate, as high as 10.5% had been reported in the GRA registry.15
Researchers reported that older adults and adults with comorbidities had an increased risk of severe COVID-19 infection, as well as poor infection outcomes and death.15,16,17,18 These observations were found in our study by means of the non-parametric analyses (Table 3), though the logistic tests nullified it. Perhaps this could be explained by our largely younger population group with a median age of 39-years-old and 66.2% were less than 50-years-old. In addition, comorbidities might have a role in contributing to recurrent infections in more than three-quarters (77.8%) of the cases (Table 1), despite having the majority of patients fully vaccinated after 1st infection (88.9%), in low disease activity/remission (61.1%), and only had low-medium steroids dose (prednisolone ≤10 mg OD) in 50% of cases. Pertinent to pregnant women with ARD, favorable infection outcomes were seen in our affected patients, as reported by GRA registry.19
A number of rheumatic disease-related factors were identified to be associated with severe disease and poor outcomes including fatality. Reportedly, disease activity and steroids especially at a dose of ≥10 mg prednisone-equivalent daily were associated with death in ARD.11,15,16 Active disease activity might have contributed to severe disease outcomes in our overall population as well as in those with complete vaccination. As many as 24% of the overall population and 19.5% of those with complete vaccination had moderate to high disease activity. The fatality could be associated with active ARD and higher steroid doses in the completed vaccination population. For example, case fatality No. 1 (Table 2) had high disease activity and used medium-dose prednisolone (30 mg once daily) prior to infection despite being fully vaccinated.
In contrast, the existing evidence showed that the use of most conventional synthetic, biologic, and targeted synthetic DMARDs does not confer an increased risk of poor outcomes in ARD patients with COVID-19 infection with the exception of rituximab.13 As a matter of fact, a higher mortality rate was reported in ARD patients who did not receive DMARD.15 Consistently, researchers showed that biologic and targeted synthetic DMARDs, particularly TNF-α inhibitor monotherapy, were associated with a lower risk of hospitalization and death.11,15,16,18,20,21 The possible explanation for the relatively favorable outcome in biologic and targeted synthetic DMARDs is their immunomodulatory (anti-cytokine) effect in a proinflammatory state associated with COVID-19 infection.22 The evidence of other IS (methotrexate, leflunomide, sulfasalazine, mycophenolate mofetil, azathioprine, cyclophosphamide, and calcineurin inhibitor) associated with high mortality rate, however, was rather conflicting.11,15,21 In this study, we found that the use of IS (all DMARDs) could be associated with better infection outcomes in both the overall population and the complete vaccination population. Likewise, the fatality could be reduced in the overall population; In fact, zero death was recorded in those who were on IS therapy. Interestingly, our patients who had biologic DMARDs (TNF-α inhibitor and rituximab), as well as IV cyclophosphamide therapy, might fare better than those on conventional synthetic DMARDs in terms of infection outcomes.
B-cell depleting therapy (BCDT) was consistently shown to be associated with poor outcomes and fatality in patients with rheumatic and non-rheumatic diseases.15,23,24,25 We had two patients who received IV rituximab therapy (regimen: 1 gm on D1 and D15) for refractory SLE with a background of medium-to-high dose prednisolone (17.5 mg OD and 50 mg OD, respectively). Both received 2 and 3 doses of COVID-19 vaccines (all were Pfizer-BioNTech, NY, USA) at the recommended timing prior to IV rituximab infusion. 1st patient contracted COVID-19 infection 3-months after IV rituximab (when she attained low disease activity), and 8-months elapsed after the second vaccine dose. She had a mild infection and made full recovery. The 2nd patient was infected 3-weeks after completion of IV rituximab, and in the 3rd month after completion of booster vaccine dose. Her SLE activity was high then. She had a moderate COVID-19 infection, requiring low supplemental oxygen (3 L/min). She recovered fully, nonetheless.
Pertinent to COVID-19 vaccination, it has been proven to be effective in reducing infection rate and preventing severe outcomes and death in the ARD population, comparable to the general population.26,27,28 The good safety profile of vaccination against SARS-CoV-2 in ARD patients was also hugely reassured by the EULAR Coronavirus Vaccine (COVAX, European Commission (EC) and Government of France) registry29 as well as in other studies.27,28 Our study showed that severe COVID-19 infection might be prevented with adequate vaccination (which is dose-dependent). Yet we could see up to 28% of patients did not get vaccinated prior to infection despite the massive nationwide COVID-19 vaccination program (commenced in March 2021). Similarly, vaccine hesitancy had been reported in other Asian nations too.30,31,32 At a worldwide scale, the estimated acceptance rate of COVID-19 vaccination was also low (67.8%) as revealed in a recently published systemic review and meta-analysis.33
) had received complete vaccination and yet developed a breakthrough infection during Delta and Omicron dominant periods (43.9% and 56.1%, respectively) despite following the recommended timing for vaccination.34,35,36 A mean time of 4.2± 1.8-months elapsed after the second dose in the primary vaccine series before the breakthrough infection occurred, similar to the report from the GRA registry (112±60-days).37 Studies in Israel and USA showed that immunosuppressed patients comprised the majority of the breakthrough infections requiring hospitalization, with some cases assuming severe and critical course.37,38,39,40,41 Data from the EULAR COVAX registry, nevertheless, showed that breakthrough infections occurred infrequently in fully vaccinated patients (0.7% in ARD and 1.1% in non-ARD).29
In general, the majority of ARD patients could generate adequate humoral response following vaccination though lower antibody titres may be expected.13,42 The waning humoral response after primary vaccination over time is the key factor in reduced vaccine effectiveness. The substantial decrease was noticed as early as week 20 (5-months) after the second dose of both mRNA (Pfizer-BioNTech, NY, USA, Moderna, MA, USA) and adenoviral vector (Oxfor-AstraZeneca, Cambridge, United Kingdom) vaccines in general population.43,44,45,46 People aged 55-years-old and older, with comorbidities and immunosuppression, in particular, had higher vaccine waning.43,45
As a matter of fact, antibody titre and neutralizing activity as well as vaccine effectiveness could be blunted in patients with immunosuppression.13,38 The use of certain IS, including BCDT, high-dose steroids, mycophenolate mofetil and Januse Kinase inhibitors, might reduce the vaccine immunogenicity.13 The researchers from the GRA registry reckoned that amongst those (fully vaccinated) hospitalized for COVID-19 infection, more than 50% of patients used BCDT and mycophenolate mofetil (40.9% and 13.6%, respectively), and the BCDT group accounted for 80% of the case fatalities (4/5).37 Similar observations were found in a smaller study in US.41 The fact that up to 75% of these affected patients had at least one comorbidity, including obesity, chronic lung diseases, and diabetes mellitus, renders them at higher risk of breakthrough infection and poorer infection outcomes.37,41
In order to prevent breakthrough infection, especially in the context of the emergence of variants of concern and subvariants, the strategy for booster vaccination has been propagated internationally and locally. The safety and effectiveness of booster (monovalent) vaccines had been well-studied,27,45,46,47,48,49 and this led to strong recommendations for ARD patients by Centers for Disease Control and Prevention (CDC) and ACR.34,50 The FDA also had recently authorized (under emergency use authorizations) bivalent mRNA vaccines as a booster dose for their better vaccine effectiveness against the current dominating omicron variant BA.4 and BA.5.50 Despite our national policy for booster doses (up to 2 doses) in high-risk groups, the vaccination rates were, however, low among the overall adult Malaysia population (68.8% for 1st booster, 2.2% for 2nd booster, as of 3/10/2022).35,51,52 Sadly, our home state (Kelantan) scored the lowest rates in the country, with 26.9% for 1st booster and 0.2% for 2nd booster.
The availability of COVID-19 monoclonal antibodies, Tixagevimab/Cilgavimab (Evusheld) in the country has, nevertheless, provided an alternative preventive measure as pre-exposure prophylaxis for ARD patients, particularly in those who received BCDT or with incomplete vaccination.51 As the evidence for the management of COVID-19 becomes more consolidated, we are fortunate to have adequate treatment options targeting various severity categories of infection, and these included antiviral agents (Nirmatrelvir/Ritonavir, Molnupiravi, and Remdesivir) and immunomodulatory therapy (Baricitinib and Tocilizumab).53
CONCLUSION
This is a small observational study we conducted in order to look into the impact of COVID-19 infection unto Asian ARD population. It showed that these patients are potentially at risk of severe infection and poor outcomes, especially in an active disease state. IS use (excluding HCQ and steroid) might contribute to better outcomes, perhaps through the immunomodulatory effect. In patients with complete vaccination, active disease and high-dose steroids could be the confounding factors. The limitations of this study, however, are a small sample size with potential biases and errors due to the sampling methods and possible under-recorded cases. We hope a nationwide study would help in better statistical analyses and inferences, such that we could use this data as a measure of preparedness for any future pandemic-scale infection.
ACKNOWLEDGEMENT
The authors would like to thank the Director General of Health Malaysia for permission to publish this manuscript.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.












