INTRODUCTION
The first event of coronavirus disease-2019 (COVID-19) pandemic in Italy was recorded on January 31, 2020, when two tourists coming from China were found to be virus-positive in Rome.1,2
On February 20, 2020, new outbreaks were detected in Codogno, Lodi Province, Lombardy region with 16 infected people, summing up to 60 on the following day and causing the first deaths soon after.3-5
Since then, the epidemic has spread throughout our country at different rates among regions, being Northern ones devastated and Southern ones somewhat spared. It mostly affected older people (40.6% of 80- to 89-year-old subjects, 29% of 70- to 79-year-old ones, 10.9% of those 60 to 69-years of age and progressively down by age classes to 0.2% for 30- to 39-year-old individuals) without significant gender differences (men=52.5%)
Under recommendations from the specific National Scientific and Technical Expert Committee, the Italian Government developed population-oriented operative provisions, and deputized the Civil Protection to organize the best possible nationwide support against the spread of infection. The Ministry of Health (MoH) and the Istituto Superiore di Sanità (ISS) provided the general population daily with details concerning contagion patterns, number of infected people staying in dedicated hospital wards, or intensive care units (ICUs) and death rate. They also recommended the most appropriate behavioral approach for infection containment with an eye to an ever-changing picture in terms of epidemiology (i.e., prevalence, incidence, and risk of spread) and clinical course, including best therapeutic strategies and lethality. Data analysis also allowed to detect any possible differences in infection features from those described in early case reports published by hospital groups involved in coronavirus outbreak in the Hubei Province and the town of Wuhan, China.6-8 One of the earliest and most frequent consequences of the pandemics’ devastating impact on health care organizations in Italy was the conversion of several hospital wards into COVID units. Most wards originally meant for internal medicine were converted in fact, as their specialists granted the high multidisciplinary competencies needed to deal with most common COVID patients of that period, i.e., those characterized by advanced age and several comorbidities. Furthermore, especially in the early phases of the pandemic, many healthcare providers (HCPs) died while trying to face such an impressive infection wave. Several frail severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2)-infected patients, including those on chronic dialysis, who, under normal conditions, would have been admitted to specialized wards, entered the above COVID units because of the high life-saving expectations placed on their internal medicine experts by hospital managers. Such an unexpected situation prompted us to write this paper devoted to the best possible strategies aimed to prevent SARS-CoV-2 infection in frail, mostly cardiopathic patients, represented mainly by those with end-stage renal disease (ESRD)/chronic dialysis, diabetes mellitus (DM) and arterial hypertension (AH).
The body of knowledge accumulated so far on the new virus, as well as to the related disease, points to the lack of any clearcut causal treatments, despite the identification of some drugs acting efficiently enough against the inflammatory storm and consequent thrombophilia precipitated by the virus. From first Wuhan results, calculated overall COVID-19 mortality was 2.3%, major risk factors being age (1.3% in 50- to 59-year-old people, 3.6% in 60-to 69-year-old ones, 8.0 % in those aged 70 to 79-years and 14.8% for octagenarians and over), cardiovascular diseases (CVD; 10.5%), DM (7.3%), chronic respiratory diseases (6.3%), AH (6%), and cancer (5.6%).6-9
Italian Data Provided by the Ministry of Health and the Higher Institute of Health
As mentioned above, the higher incidence of the disease in older ages is further supported by Italian data from 522,521 individuals recorded on April 27, 2020, (Table 1), according to which 2.0% cases were critically ill, 17.5% had a clinically severe disease while 35.5% had a mild one, 16.3% had very few, and 15.4% had illdefined symptoms. 13.3% of subjects were entirely asymptomatic and represented a hazardous source of infection because they did not raise suspicion and, as confirmed by a population screening from Tuscany and by Diamond Princess cruise ship results, were mostly younger and moved around quite easily.10,11,12
| Table 1. Most Common Comorbidities Observed in SARS-CoV-2 Positive Deceased Patients. Data Available on April 23th, 2020 |
|
Diseases
|
N |
%
|
| lschemic heart disease |
562
|
27.5
|
| Atrial fibrillation |
450
|
22.0
|
| Heart failure |
328
|
16.1
|
| Stroke |
228
|
11.2
|
| Hypertension |
1410
|
69.1
|
| Type 2-diabetes |
647
|
31.7
|
| Dementia |
307
|
15.0
|
| Chronic obstructive pulmonary disease (COPD) |
350
|
1Z 1
|
| Active cancer in the past 5-years |
328
|
16.1
|
| Chronic liver disease |
81
|
4.0
|
| Chronic renal failure |
431
|
21.1
|
| Dialysis |
43
|
2.1
|
| Respiratory failure |
110
|
5.4
|
| HIV Infection |
5
|
0.2
|
| Autoimmune diseases |
76
|
3.7
|
| Obesity |
249
|
12.2
|
| Number of Comorbidities |
| 0 comorbidities |
74
|
3.6
|
| 1 comorbidity |
294
|
14.4
|
| 2 comorbidities |
431
|
21.1
|
| Source:13 |
The ISS provided the list of main co-morbidities identified in deceased people as of April 23, 2020 (Table 1), among which DM and all-cause nephropathies displayed the top prevalence rates (31.7% and 23.2%, respectively) immediately lower than CVD and AH. Even though the above-reported rates allowed no differentiation between unfavorable clinical course and susceptibility to infection, the latter seemed to get the lead.13
Another potentially dangerous vector of infection, especially for frail patients, is represented by health care providers (HCPs) when missing adequate individual protection devices (IPD) and infection containment procedures,12 who are at highrisk for contagion and death themselves: as of April 29, 2020, 151 physicians died, as well as, 34 nurses, 18 health workers and 13 pharmacists Table 1.14
Factors Favoring the Contagion
All of the above, namely the vast extent of asymptomatic infected people meeting healthy individuals, advanced age, chronic diseases, and promiscuity, represent explosive mix. Where is it mostly expected to happen? Wherever people with ESRD are mostly concentrating, i.e., in internal medicine wards and, even more, in dialysis units, of course. In such areas, aggregated chronic diseases profoundly affect patients, who are mostly characterized, in turn, by older age and an altered immune response,9,10 mostly impacting just on those T-cells (mostly T-helper and NK cells) which play a significant role against COVID-19 disease severity.15 On the other hand, uremia itself associates with immune dysfunction,16 thus potentially facilitating SARS-CoV-2 infection, too.17,18 Room logistics might also amplify the risk of getting infected. Several patients undergoing dialysis at the same time are usually spending at least 4-hours, i.e., the full duration of a therapeutic session, together with dedicated health care professionals.19-21
Fragile Population: Dialysis Patients with and without Diabetes
Why are we so interested in patients with ESRD and/or on dialysis for COVID-19 risk? Because DM is highly prevalent among patients on dialysis and the vast majority of the latter have diabetic nephropathy (DKD).22,23 DM patients run a 12-fold risk of entering dialysis (130 per 10,000 persons) as compared to those without DM (11 per 100,000) and have a lower 3-year survival rate than metabolically healthy patients (55% vs. 68%). Moreover, people with DM with a history of arterial hypertension, previous myocardial infarction, congestive heart failure, or peripheral vascular disease have a higher likelihood of starting dialysis earlier than those free of the complications above and significantly increase healthcare costs.24 To summarize, DM and ESRD, when taken together, might act as a severe life-threatening association.
However, the few data coming from larger groups of patients observed in China during the epidemic, despite being only partially concordant on infection rates, do not support the hypothesis that dialysis might increase the risk for contagion. One paper reported on a 13.9% COVID-19 infection prevalence (33 out of 237) and a 16.2% mortality rate (presumably not only due to COVID-19) in a dialysis unit with four infected individuals among physicians.25 The other one, instead, reported a 2.5% COVID-19 infection prevalence (5 out of 201) without any severe lung complications or deaths.9 It is not possible to know how many had DM nor which was their treatment regimen, yet, despite the abovementioned unfavorable conditions, infection rates showed to be rather low.
Media-reported data on dialysis units from Lombardy, i.e., the most affected region in Italy, indicate that the first dialysis treated case occurred in Lodi, i.e., the “red lockdown area”, where a dedicated contagion prevention protocol was adopted. According to the Italian Nephrology Society (INS), the incidence of COVID-19 infection within dialysis units was 16% in China vs. only 2% as a mean in Italy reflecting, however, meager rates in Southern regions vs. 7% in Lombardy (where 14% was recorded in Cremona Hospital and 10% in other centers). Data has then evolved quickly.26
Dialysis and Diabetes
The fact that DM did not appear in charts from people hosted in dialysis units during the epidemic either in China or in Italy is easily explained by the severe emergency striking ICUs which all of a sudden were flooded with patients requiring mechanical ventilation and further burdened with the lack of suitable treatment strategies against the unknown disease. Therefore, it is most likely, albeit hard to prove, that care teams initially committed only to the maintenance of vital functions, including respiration and, in the case of ESRD patients, to the prevention of the spread of infection. This hypothesis is further supported by the observation that several groups provided mostly non-structured recommendations on infection containment within dialysis units.27-34 Two papers come up with structured suggestions that are a bit too general, though, and hardly adapted to different specific hospital unit needs.30,35
Interfering Factors
Problems encountered by patients with ESRD and DM represent much more than the sum of individual diseases. Moreover, insulin has always been considered the treatment of choice in DM patients on dialysis but is often responsible for hypoglycemic events requiring a substantial dose reduction over time.36,37 Indeed, despite being mostly asymptomatic, hypoglycemia (HYPO) occurs quite often in dialyzed people as a result of various factors including not only oral hypoglycemic agents or insulin but also dietary errors/prolonged fasting/chronic malnutrition, malignancies, heart/hepatic/ renal failure, adrenal or thyroid deficiency, beta-blockers, or other drugs.38 Moreover, nephrologists know very well that ESRD entails a significant intra-and between day glycemic variability (GV) due to intermittently enhanced insulin clearance rate and that HYPOs contributing to GV are bound to increase further an already high cardiovascular risk (CVR).39-44
An interfering factor also adds to the above: different mechanisms might affect COVID-19 expression concerning some drugs other than insulin eventually used by individuals with ESRD. Based on experimental results still awaiting clinical confirmation, DPP-4 inhibitors seem to have a protective role,45 while pioglitazone, GLP1-receptor agonists, and insulin itself might even favor infection per se or related disease severity.46 All this deserves further investigations in the human to avoid unnecessary and potentially hazardous drug withdrawal, as observed with other molecules in the early contagion stages: ACE-inhibitors and angiotensin-II receptor blockers were discontinued by some patients without any GP consultation, due to a supposed respiratory failure enhancing potential, which scientific societies soon denied.45,46
In any case, DM treatment has to be optimized in the presence of both SARS-CoV-2 infection and any other concurrent disease including renal failure47 to prevent HYPOs and GV per se from further increasing CV risk, mainly when corticosteroids are utilized, according to some COVID-19-specific protocols, which are known to worsen prognosis trough impaired glucose control.48
Treatment Strategy
SARS-CoV-2 pandemic still raises too many doubts about the best possible treatment strategy for DM patients on dialysis. Three of them require an urgent reply in our view: (i) are these patients more susceptible to SARS-CoV-2 infection?; (ii) is their clinical course more severe?; (iii) which procedures might minimize contagion risk?
Reliable answers to the first two questions may only be given when large enough case series are finally available from the literature. However, lower inflammatory cytokine levels have been reported in dialyzed patients so far,49 which might account for the less severe clinical course of COVID-19 than expected.
Aim of the Prevention Protocol
We were particularly interested, however, in the question concerning procedures eventually minimizing contagion risk per se and tried to answer that by looking at the results of the infection containment protocol we adopted in the 18 private Dialysis Centers from the Nefrocenter Consortium contracted to the Italian NHS in favor of Campania region patients.
Protocol
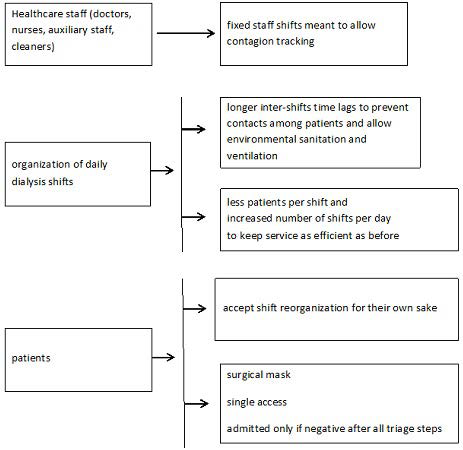
The protocol, as defined by us according to INS recommendations50,51 and to Chinese experience,25 has been operating since the epidemic outbreak in Lombardy, i.e., February 20, 2020, to limit the spread of contagion among both patients and HCPs directly or indirectly involved in dialysis-related activities. It included the following steps: (i) a daily and weekly telephone call-based pre-triage (Figure 1); (ii) patient transfer back and forth from home to the dialysis unit (Figure 2 and Table 2); (iii) treatment room activity remodulation (Figure 3) involving admission of fewer patients at the same time (10 per session; range 3-18), and 25 to 40% lower dialysis unit allowance per shift. Table 3 describes IPDs used by our health care personnel. Working environment and patient-devoted shuttle bus sanitization methods took advantage of a wide-spectrum biocidal solution (i.e., Evolyse Strong®; hydrogen peroxide+silver sulfate) nebulized through the MEDIBIOS Basic device (i.e., a readyto-use solution diffuser), as well as, of alcohol and chloride-based solutions. In the event of suspected SARS-Cov-2 infection, oropharyngeal swabs were used for viral ribonucleic acid (RNA) Polymerase chain reaction (PCR)-based detection, according to specific modalities defined by the Italian MoH (Table 4). The study was conducted in accordance with the Helsinki Declaration 1975 and subsequent amendments, and was formally approved by the Ethics Committee of the University of Campania “Luigi Vanvitelli” on April 30, 2020 (protocol n. 1394). The data were processed anonymously according to good clinical practice guidelines and no ptient started the study before signing informed consent.
Figure 1. Telephone Call-Based Triage before Transfer to the Dialysis Center
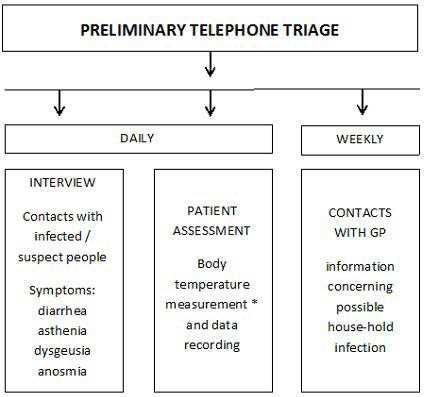
Immediate reporting to the GP of suspected cases according to procedures established by the MoH. * traditional thermometer
Figure 2. Triage Dialysis Center Transfer as Carried out by Specifically Trained Drivers to Check whether or Not Patients’ Telephone Statements are Correct, and as Repeated by the Health Personnel upon Arrival to the Filter Area Outside the Dialysis Unit
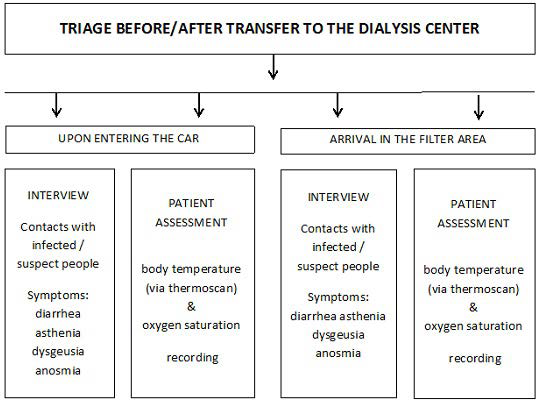
Patients’ family members are not allowed. Accepted oxygen saturation is ≥92%, due to expected high average age, as well as, COPD comorbidity. Upon infection suspicion, Immediate reporting to the GP follows, according to MoH procedures, and transfer to COVID-19 dialysis center.
| Table 2. Check-list of Actions to be Taken by Dialysis Center Personnel Involved in Patient Transfers |
|
ACTIONS
|
|
1
|
Acceptance of less patients onto the shuttle bus |
|
2
|
Sanitization and ventilation of the shuttle bus at each transfer (dedicated personnel) |
|
3
|
Use of sanitizing agents recommended by national guidelines |
|
4
|
Single sanitization procedure recordings for purpose of traceability |
|
5
|
Bus windows kept opened throughout transport breaks |
Figure 3. Remodeling of Dialysis Activity to Contain the Spread of SARS-CoV-2
| Table 3. Prevalence of Each Risk Factor of Metabolic Syndrome at Baseline |
|
Staff
|
PPE |
Notes
|
| Driver |
disposable gown, surgical mask, gloves, sanitizing gel |
|
| health workers A |
surgical mask, protective glasses, gloves, water repellent gowns |
admitted to the dialysis room only after completing all triage steps |
| health workers B |
protective filter masks, protective goggles/ visors, gloves, water repellent gowns, boots |
in isolation |
| Other workers |
Surgical mask, gloves, sanitization gel |
|
| *Legend: health workers A: staff engaged in assisting patients with mild respiratory symptoms but suspected of SARS-CoV-2 infection; health workers B: staff assisting patients with suspected or confirmed SARS-CoV2 infection. |
| Table 4. General Characteristic of Subjects on Chronic Dialysis (n=852) |
|
Characteristics
|
Values
|
| Sex (M/F) (n) |
59.9/40.1
|
| Mean Age (+SD) (years) |
|
| BMI (kg/m2) (range) |
23.9 (18.7-27.5)
|
| Type 2 Diabetes Mellitus , n (%) |
315 (37%)
|
11.2
|
| On insulin, n (%) |
252 (80%)
|
69.1
|
| On other drugs, n (%) |
63 (20%)
|
31.7
|
|
Comorbidities
|
With DM |
Without DM
|
| Cardio-vascular complications* |
52%
|
43%
|
| Stroke |
23%
|
19%
|
| Arterial Hypertension |
73%
|
70%
|
| Chronic Obstructive Pulmonary Disease (COPD ) |
24%
|
22%
|
| Active cancer in the past 5 years |
0.3%
|
–
|
| Chronic liver disease |
18%
|
22%
|
| Respiratory failure |
16%
|
18%
|
| HIV infection |
– |
–
|
| Autoimmune diseases |
–
|
–
|
| Obesity |
–
|
–
|
| Number of Comorbidities** |
| 0 comorbidities |
3%
|
6%
|
| 1 comorbidity |
14%
|
21%
|
| 2 comorbidities |
39%
|
35%
|
| 3 comorbidities and over |
44%
|
38%
|
* Ischemic heart disease, Atrial fibrillation, Heart failure
**in addition to diabetes and ESRD |
All the subjects enrolled signed the informed consent and the protocol was approved by the Scientific and Ethical Committee of the University of Campania “Luigi Vanvitelli” on April 30, 2020 (protocol n. 1394).
METHODS
At the moment, all the 381 people working at the 18 dialysis centers (i.e., 93 medical doctors, 151 nurses, 20 head-nurses, 48 health workers, 54 car/bus drivers, 15 other employees) and 825 patients, of whom 315 classified as having T2DM, were defined as suspected or positive according to the protocol mentioned above. The observation ended on June 30, 2020. The results were expressed as average±SD or %. Observed differences were tested by the analysis of variance for repeated measures (r Analysis of variance (ANOVA)) supplemented by a two-tailed Student’s t-test for parametric variables and by the Mann-Whitney U test with 95% confidence intervals (CI) for nonparametric variables. The test chi-square with Yates correction or Fisher Exact test was used to compare categorical variables. A p<0.05 was chosen as the least acceptable statistical significance level. All the evaluations were performed using the Statistical Analysis System (SAS) Program (Release 9.4, SAS Institute, Cary, NC, USA).
RESULTS
Subjectst details are provided in Table 4: 89% of individuals with Type 2 diabetes (T2DM) were on insulin, with a mean 8.7% hemoglobin A1c (HbA1c) level (range 6.2-9.9%) and a very high comorbidity rate, yet similar to those without DMT2. Notably, despite the high prevalence of obesity among T2DM patients worldwide, none of them were obese.
Out of 40 suspected people, only two were swab-identified as COVID-19 positive among patients, none among health personnel.
One of the virus-positive patients was a 58-year-old insulin-treated woman, with an HbA1c 7.9%, and having only AH as comorbidity and recovering after a 16-day home-based treatment period. At the same time, the other was an 84-year-old woman having arterial hypertension, ischemic heart disease, and poor glucose control (HbA1c=9.2%) despite intensive insulin treatment, who died of respiratory failure after ten days spent in the ICU.
DISCUSSION AND CONCLUSION
The shallow contagion rate (0.34% patients; no spread to HCPs) witnesses in favor of the timeliness and efficacy of adopted protocol, also in consideration of the fact that infection most probably occurred outside dialysis centers, the two positive women were not young and, finally, the deceased one had two deadly conditions, i.e., severe illness and poorly controlled T2DM.
An environmental factor might have also positively influenced our results, as Campania is one of the least infected Italian areas where, for instance, based on our data, people with T2DM have a low infection risk (0.11%).13,52 However, what we found is in line with the literature, according to which, despite being intrinsically frail, patients on dialysis are at lower risk for infection independently of having T2DM or not.
We are aware that, despite being large enough, our case series of 252 insulin-treated T2DM subjects on dialysis does not necessarily reflect what occurring at the national level, so further studies in the field are warranted to validate our data.
However, we hope our results may at least open a window on the SARS-CoV-2/T2DM/ESRD triad and offer a suitable solution for contagion containment procedures to be adopted under similar conditions by any internal medicine o specialized units involved in hospital care of ESRD patients during COVID-19 pandemic.
ACKNOWLEDGEMENTS
We acknowledge the logistic support of Nefrocenter Research Network & Nyx, research startup, Naples, Italy.
INFORMED CONSENT
Written informed consent was obtained from all participants before enrollment.
FUNDING
The study did not have a sponsor/funder.
AUTHORSHIP
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
AUTHOR’S CONTRIBUTION
SG, ES, CR and FS created the paper and wrote it. CA, TDC, critically read the paper. All have complied with data collection, critically assessed the results, and approved the final text. All collaborators critically read and approved the final text.
STUDY INVESTIGATORS
For full investigator list see the Appendix
COMPLIANCE WITH ETHICS GUIDELINES
This study was conducted in conformance with good clinical practice standards. The study was led in accordance with the Declaration of Helsinki 1975, as revised in 2013. Written informed consent was obtained from all participants before enrollment.
DATA AVAILABILITY
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.








