INTRODUCTION
A two-day meeting to discuss the development of International Standards (ISs) for Oral Whole Cell Killed Cholera Vaccines (OCVs) took place in Seoul, Republic of Korea, from 17-18 May 2018. The conference was organized by the International Vaccine Institute (IVI) with assistance from National Institute of Biological Standards and Control (NIBSC) and funded by the Bill and Melinda Gates Foundation (BMGF). A total of 45 delegates representing vaccine manufacturers: EuBiologics Co., Ltd., Republic of Korea; Hilleman Laboratories, India; Incepta Vaccine Ltd., Bangladesh; Shantha Biotechnics Pvt. Ltd., India; Vabiotech, Vietnam; and Valneva, Sweden; Research Institutions: IVI, Republic of Korea; icddr, b, Bangladesh; Health Canada, Canada; University of Gothenburg, Sweden; NIBSC, Great Britain; National Regulatory Agencies (NRAs): Korean Ministry of Food and Drug Safety, Re-public of Korea; ANSM: French National Agency for Medicines and Health Products Safety, France; Bangladesh Association of Pharmaceutical Industries, Bangladesh; WHO-RSS, Switzerland; and independent experts met to discuss and share experiences on in vitro and in vivo potency assays for batch release of OCVs, more specifically the lipopolysaccharide (LPS) inhibition ELISA. The LPS inhibition assay is used by both manufacturers and NRAs and the meeting set out to shape consensus on the specification of reagents for LPS Inhibition ELISA to be made into candidates for WHO International Standards (ISs).
The four objectives of the meeting were: 1) Review the potency assays currently used for batch release of OCVs; 2) Propose a set of candidate ISs and procedures to determine their suitability for the LPS Inhibition ELISA; 3) Discuss challenges of using LPS Inhibition ELISA to assess vaccine potency as part of an application for controlled temperature chain (CTC) use and for the determination of shelf life; and 4) Discuss the feasibility to develop a suitable in vivo potency assay for batch release of OCVs. The two expected outcomes of the meeting were: 1) Agree on a consensus for a set of candidate ISs for the LPS inhibition ELISA and 2) Determine the need for and feasibility of an in vivo potency assay. Here we summarize the discussions and main conclusions of the meeting.
Cholera: The Disease and Current State of Vaccines
Cholera is an acute, rapidly-dehydrating diarrheal disease transmitted through water or food contaminated with the bacterium Vibrio cholerae O1 or V. cholerae O139. Approximately 1.3 billion people are at risk for infection, and an estimated 1.3-4 million cases of cholera and 21,000-143,000 deaths occur globally each year.1 About 47 countries globally are at risk of cholera2 and in 2016, 54% of reported cases were in Africa, 13% in Asia and 32% in Hispaniola.3 Cholera is a disease of displacement and poverty primarily affecting people living in areas with poor access to clean drinking water, and inadequate sanitation and hygiene. While the long-term solution for cholera control lies in universal access to safe drinking water and adequate sanitation, the combination with a targeted cholera vaccination program with OCV would likely yield cost-benefit outcomes. The WHO recommends the use of these vaccines as part of an integrated strategy to control cholera outbreaks and contain the infection.1
Currently, three OCVs have been prequalified by the WHO and are available for purchase on the global market; Dukoral® (Valneva, Sweden), Shanchol™ (Shantha Biotechnics, India) and Euvichol® and Euvichol-Plus® (EuBiologics, Republic of Korea). The immunogenicity of ShancholTM and Euvichol® was found to be comparable.4 In addition, mORC-Vax™ (Vabiotech, Vietnam) and the live attenuated vaccine Vaxchora (PaxVax, United States) have obtained a national licence. Two inactivated vaccines Cholvax (Incepta Vaccine, Bangladesh) and Hillchol (Hilleman Laboratories, India) and a live attenuated cholera vaccine 638 (Finlay Institute, Cuba) are in clinical trials.5,6 Comprehensive information of current OCVs is given in Table 1.
| Table 1. Oral Cholera Vaccines Currently Manufactured and in Development |
|
Vaccine
|
Dukoral® (1) |
Shanchol™ (2) |
Euvichol® (3) |
mORC-Vax™ (4) |
VAXCHORA™ (5) |
Cholvax |
HILLCHOL |
Vibrio cholerae 638
|
|
Manufacturer (Country)
|
Valneva (Sweden) |
Shantha Biotechnics Pvt. Ltd (India) |
EuBiologics Co., Ltd.(Republic of Korea) |
VABIOTECH (Vietnam) |
PaxVax (United States) |
Incepta Vaccine Ltd (Bangladesh) |
Hilleman Laboratories (India) |
Finlay Institute (Cuba)
|
|
Components
|
Killed V. cholerae O1 and rCTB |
Killed V. cholerae O1 and 0139 |
Killed V. cholerae O1 and 0139 |
Killed V. cholerae O1 and 0139 |
Live attenuated V. cholerae O1 |
Killed V. cholerae O1 and 0139 |
Killed V. cholerae O1 Hikojima |
Live attenuated V. cholerae O1 |
| Age |
≥2 yrs |
≥1 yr |
≥1 yr |
≥1 yr |
18-64 years |
n/a |
n/a |
n/a
|
|
Buffer
|
Bicarbonate buffer |
Not required |
Not required |
Not required |
Bicarbonate buffer |
Not required |
Not required |
Not required |
| Country licensure |
1991 |
2009 |
2015 |
1997 |
2016 |
n/a |
n/a |
n/a
|
|
WHO PQ
|
2001 |
2011 |
2015 |
n/a |
n/a |
n/a |
n/a |
n/a
|
1) Dukoral® product information: http://www.who.int/immunization_standards/vaccine_quality/117_Dukoral_PI_updated_2012-07-05.pdf?ua=1
2) Shanchol™ product information: https://extranet.who.int/gavi/PQ_Web/(X(1)S(15z42ckmojpm14g5wgtiisdd))/PreviewVaccine.aspx?nav=0&ID=249
3) Euvichol® product information: http://www.who.int/immunization_standards/vaccine_quality/pq_298_euvichol_1dose_eubiologics_PI.pdf?ua=1
4) mORC-Vax™ product information: https://www.stopcholera.org/sites/cholera/files/comparison_of_the_killed_ocv_products.pdf
5) VAXCHORA™ product information: https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM506235.pdf |
Despite the high burden of cholera in many parts of the world, the demand for OCVs has historically been low. Since 2013, the International Coordinating Group (consisting of the International Federation of the Red Cross and Red Crescent Societies, Médecins sans Frontières, United Nations Children’s Fund and the WHO) and the Global Task Force on Cholera Control is managing a stockpile made up of Shanchol™ and Euvichol® (added in 2015). Both these vaccines have been used with great effect to control cholera in outbreaks, humanitarian crises, and in endemic settings.7,8,9,10 With new affirmative recommendations from WHO, the availability of the stockpile, and the production of new and more affordable OCVs, the demand for OCVs has increased, with shipments from the stockpile steadily increasing each year.11 To date, around 25 million doses of OCV have been deployed through campaigns in 19 different countries.12,13,14 The WHO has launched an ambitious plan: Ending Cholera-A Global Roadmap to 2030, which proposes a 90% reduction in cholera deaths by 2030 through preventive OCV campaigns and improvements in WASH. The successful rollout of this plan will require substantial increases in available vaccine supply.
The Need for Standardization of the Potency Assay for OCV Batch Release
V .cholerae strains can be differentiated based on the serogroup -specific part of the Lipopolysaccharide (LPS) O antigen also called O Polysaccharide (OPS) present on the surface of the bacterium. Over 200 serogroups of V. cholerae have been identified, but only serogroups O1 and O139 are known to cause epidemic cholera.15,16 The O1 serogroup, which currently accounts for >99% of all cholera cases globally, is further divided into two biotypes (El Tor and Classical) and subdivided into two serotypes, Ogawa and Inaba.17 The difference between the Ogawa and Inaba serotypes lies only in the terminal perosamine sugar of the O1 OPS which is methylated in Ogawa strains and not methylated in Inaba strains (Figure 1).18,19 Although Inaba and Ogawa serotypes can be serologically distinguished, they exhibit strong immunologic cross-reactivity such that most of the immune response induced by vaccination with the Inaba O1 LPS recognizes Ogawa O1 LPS and vice versa. Hikojima is the third O1 serotype, which is found to be an unstable phenotype. It expresses O1 LPS with a reduced presence of methylated perosamine in its O1 OPS. Therefore, Hikojima isolates present both Ogawa and Inaba O1 LPS structures on their surface.13 For vaccine purposes, genetically modified strains of the El Tor and Classical biotypes have been engineered to constitutively express the Hikojima O1 LPS.20
Figure 1. Structure of the OPS of Vibrio cholerae O1, Serotypes Inaba and Ogawa
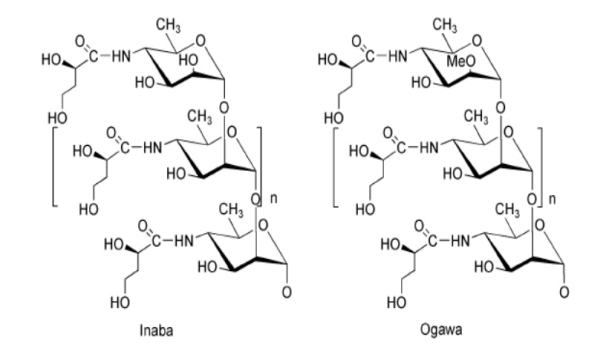
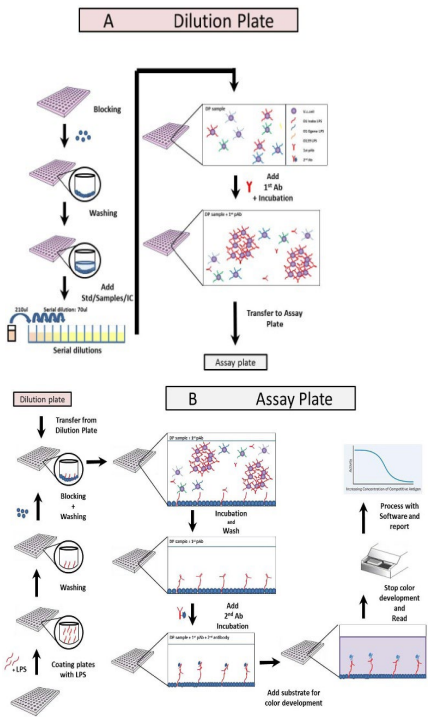
Even though immune protection against V. cholerae is mediated by locally produced IgA anti-V. cholerae antibodies in the intestine, serum vibriocidal antibodies are associated with immunity to cholera.21,22 The main protective antigen of OCVs is the O LPS which is also the target antigen for vibriocidal antibodies.23 Thus, the potency of OCVs can be determined by the amount of O LPS antigen per standard human dose as measured by the LPS inhibition ELISA (Figure 2). To pass, a batch of OCV must contain a minimum amount of V. cholera O LPS and by association a minimum number of bacteria as specified in the marketing authorization. The LPS inhibition ELISA uses anti-O LPS antibodies and a reference vaccine to quantitate the amount of O LPS present on the inactivated bacterial cells; either in the monovalent cell bulks (drug substance, DS) or in the final lot (drug product, DP) of the test vaccine. Manufacturers use the assay to assess the quality of the vaccine manufacturing process and NRAs use the assay to establish the potency of the OCV batches prior to release for the market.
Figure 2. Procedure for the LPS Inhibition ELISA
Determination of vaccine stability forms a crucial part of the evaluation of licensed vaccines to ensure that these remain potent and efficacious until the end of their shelf-life when stored under approved conditions. For OCVs, storage conditions are at the traditional cold chain temperature of +2 ° C to +8 ° C. However, for delivery of OCVs in the field maintaining a cold chain can be very challenging, especially in the final leg of the distribution. Cholera outbreaks often occur in isolated rural areas or slums, where refrigeration facilities are severely limited or absent. To extend immunization programs in regions with cold chain challenges, the WHO has developed the “controlled temperature chain” (CTC) program.24 Under the CTC program, vaccines that have been evaluated using appropriate methods by competent authority are authorized for a planned temperature excursion at 40 ° C for a minimum of 3-days.25 For vaccines that cannot meet the minimum CTC program requirements, the same statistical methods can be used to determine the excursion potential of the product, but it cannot be approved with a CTC stability indication. Due to the challenges faced when distributing OCVs, these are on the WHO priority list of vaccines to be approved for CTC, and manufacturers are hence encouraged to seek CTC approval for their OCVs. For this approval, manufacturers must demonstrate the stability of their products which can be done using the LPS inhibition ELISA.
The LPS inhibition ELISA is used by several OCV manufacturers and NRAs for potency testing, batch release and stability indication and this has resulted in variations in the procedure and reagents. To ensure sustainable access to high-quality reagents and to support harmonization of LPS inhibition ELISAs performed in laboratories of manufacturers, NRAs and researchers in different parts of the world, specific biological reference materials are a necessity. The WHO and the WHO Expert Committee on Biological Standardization (ECBS) propose and endorse such reference materials called WHO international standards (IS). ISs are used as ‘primary standards’ for assays or procedures, where specific biological activity is measured. NRAs and manufacturers can use the IS to validate their in-house assays and to calibrate their in-house working standard or national reference reagent. Currently, no ISs are available for the LPS inhibition ELISA. However, the need for specific ISs to harmonize and improve the robustness of the LPS inhibition ELISA is well recognized by the different stakeholders within the global OCV community, and in October 2017, ECB endorsed the proposal to develop reagents of the LPS inhibition ELISA into ISs.26
Pre-meeting Questionnaire on in Vitro Potency Assays for Batch Release of OCVs
Manufacturers were asked to share comprehensive information about their assays through a questionnaire sent out prior to the meeting. The results were presented and discussed on the first day of the meeting. All manufacturers produce OCVs consisting of inactivated cells, 4 out of 6 produce bivalent vaccines containing O1 Inaba, O1 Ogawa, and O139, one manufacturer produces a monovalent vaccine containing O1 Inaba and O1 Ogawa and one manufacturer produces a monovalent vaccine containing only O1 Hikojima. All manufacturers use the LPS inhibition ELISA for quantification of the O1 LPS in their vaccine, and 5 out of 6 use the assay for release of both DS and DP. An inventory of ELISA procedures showed concordance regarding the type of ELISA used with some variations for essential reagents, in particular, variations in the O1 LPS and O139 LPS antigen used for coating and type and specificity of anti-LPS O1 and anti-LPS O139 antibodies (monoclonal or polyclonal). The specifications of reference vaccines showed some variation for monovalent cell bulks and final lots. These observations strengthen the need for ISs that cover essential reagents like O LPS antigens for coating and anti-O LPS antibodies for detection. All participants agreed that they would benefit from the availability of international reference materials for the LPS inhibition ELISA.
Candidate International Standards Characterization and Suitability in the LPS Inhibition ELISA
On the second day of the meeting, all delegates (manufacturers, representatives from NRAs and experts in the field) were invited to discuss and propose the specification of LPS inhibition ELISA reagents.
In concordance with the outcome of the questionnaire, all delegates agreed that the LPS inhibition ELISA should be used as a potency assay to measure the amount of O LPS in both DS and DP and during the discussion it became clear that the format of the assay varied among manufacturers as well as NRAs. Indeed, manufacturers use the results of their in-house assay to assess whether the DS or drug product DP meets the specification set out in the marketing authorization. Therefore, it was agreed that LPS inhibition ELISA procedures should not be harmonized or prescribed. Instead, a collaborative study to evaluate and validate the suitability of the candidate ISs in various in-house ELISA formats should be conducted. By thorough discussion, a consensus was reached and the preferred candidate ISs for the LPS inhibition ELISA are: 1) purified O1 Inaba LPS, O1 Ogawa LPS, and O139 LPS as coating reagents. Initially, only LPS from O1 Inaba and O139 were agreed upon, however many delegates proposed to include O1 Ogawa LPS and this was accepted by the organizing committee and experts in the field; 2) an anti-O1 LPS monoclonal antibody recognizing the shared component of O1 PS of Inaba and Ogawa LPS and an anti-O139 LPS monoclonal antibody as primary antibodies for detection of V. cholerae LPS. The anti-O1 monoclonal antibody should react with both Inaba and Ogawa LPS and 3) Reference vaccine: a mixture of whole killed cells of V. cholerae O1 Inaba, O1 Ogawa, and O139. Preferably, this should be a GMP batch donated by a company that has been prequalified by WHO. The batch will be filled and freeze-dried (optional) at NIBSC, reflecting one single human dose per ampoule.
It was agreed that for optimal storage, the O LPS antigens and monoclonal antibodies should be freeze-dried and stored at -20 o C, which is the default approach for production and storage of ISs at NIBSC. These conditions will ensure products are stable for a very long time. For the reference vaccine, the suggestion was to produce liquid fills and store these at or below -20 o C. A proportion of this material will also be freeze dried. Frozen and freeze-dried reference vaccine should then be evaluated in a small comparative study to assure compatibility and validated by a multi -laboratory (collaborative) study.
Two aspects raised considerable discussion among the manufacturers and these were related to the specification of the vaccine in terms of content and units displayed on the label of the final lot or DP. Licensed vaccines contain V. cholerae serogroup, O1 Inaba and Ogawa. If a monoclonalanti-O1 LPS antibody is used, then quantification of Inaba and Ogawa cells can only be done in the monovalent DS stage prior to mixing, and not in the final DP, when both serotypes are present. Hence these units would be related to anti-O1 LPS activity only. Quantitation of both serotypes in the final lot is only feasible if cross-adsorbed polyclonal anti-O1 Ogawa LPS or anti-O1 Inaba LPS sera are used. Labeling the content of both serotypes in the DP will, therefore, pose a challenge and may be impractical.
Licensed OCVs have a specified label value based on successful clinical trials and from the discussion, it also became clear that manufacturers use different units to specify the amount of V. cholerae LPS or cells in the DP, including colony-forming units, micrograms or ELISA units. Although suggestions were made to ascertain equivalence between the specifications of existing licensed OCVs or OCVs currently in clinical trials, it is clear that candidate ISs will only be available sometime after licensure of OCVs, their entry into the market and their use in public health programs. Thus, it was decided that interference with label values of licensed OCVs is unnecessary and counterproductive, therefore efforts of a collaborative study should be directed at verifying the suitability of candidate ISs in various in-house LPS inhibition ELISAs (as mentioned above). The outcome of the study would thus provide information on the quality, consistency, and commutability of inhouse LPS inhibition ELISAs used for batch release.
Finally, the different software used to analyze ELISA data and their specifications were briefly discussed. Because different programs are used successfully in-house, it was decided that no recommendation for software should be made. However, it could be helpful to compare different software to ensure equivalence of analyses carried out. All delegates agreed that ideally, the software used should be validated for its purpose and dedicated to the analysis of the complete dose response.
Use of the LPS Inhibition ELISA to Assess Potency as Part of an Application for CTC Use and Determination of Shelf Life
One expert presentation on the stability evaluation25 for a CTC label was followed by a discussion among delegates. The discussion and results from the questionnaire showed that all manufacturers use the LPS inhibition ELISA to monitor the stability of OCV during storage, but only one manufacturer applied the LPS inhibition ELISA for CTC labeling. From the meeting, it became clear that many aspects need to be taken into consideration for stability analysis. The expert presentation emphasized the importance of using appropriate assays and specifications in stability studies. While certain manufacture’s data suggest that their LPS inhibition ELISA may be stability indicating, in general, further data is required to demonstrate, with a high degree of confidence, the extent to which these ELISAs sufficiently represent the protection offered by a vaccine. For example, what other antigens may be involved in protection? Or, is there compelling data to suggest that such considerations are not relevant? It was noted that to address these issues, they should be included in a CTC application. It was also emphasized that given the extreme challenge that CTC conditions represent, it is critical that CTC assessments are well supported, such that neither the vaccine recipient nor that CTC program is put at risk. If one assumes that a compelling case could be made that supports LPS inhibition ELISAs as the basis for a CTC approval, ideally, serotype-specific LPS content should be monitored to establish the stability characteristics for each OCV formulation. The specifications for LPS content are product specific and based on the key quality attributes of final lots that are representative of those that have been demonstrated to be safe and efficacious in clinical use. If a decrease of LPS content is observed over the incubation period, then different specifications for the number of LPS should be established for batch release and end of shelf-life of the DP. Furthermore, the specification at the end of shelf-life should be linked to lots demonstrated to be safe and effective/ immunogenic in clinical trials, to ensure that all commercial lots are effective at the end of shelf life (including the planned CTC excursion). The challenge to measure serotype-specific LPS content in the final drug product was discussed since reagents are currently not available to quantitate O1 Ogawa or O1 Inaba epitopes separately. The consensus from the meeting was that it is the responsibility of each manufacturer to demonstrate that the assay used to assess the stability of the OCV is stability indicating when seeking CTC approval.
Feasibility of a Relevant in Vivo Potency Assay for Batch Release of OCV
The relevance of in vivo potency assays for batch release of OCVs was discussed on the second day following three presentations. According to the questionnaire, none of the manufacturers used or knew of an in vivo potency assay for batch release of OCVs. This was reflected in the presentations
which showed that in vivo potency assays only have had a meaningful role in pre-clinical trial studies of inactivated and live attenuated OCVs. Although, in vivo potency models can be used for OCVs to evaluate efficacy and identify relevant biomarkers (e.g. the parental and mucosal immune response), even at this stage there are clear challenges to these models including lack of translation of efficacy of OCV in the rodent model to clinical studies, the high variability and high cost. This makes these animal models unsuitable for quality control. NRAs emphasized their main concern regarding the use of in vivo models for batch release, in that this approach is going against the reduction, refinement, and replacement of animal use (the 3Rs) when evaluating DP for batch release. The consensus among the delegates was that in vivo potency assays are useful for pre-clinical studies, but these are unsuitable for batch release.
Next Steps
The meeting reached a consensus of which reagents of the V. cholerae LPS inhibition ELISA to be developed into ISs. The outcome is broadly in agreement with the endorsement of by WHO ECBS.19 The next steps that are needed to produce the candidate ISs are:
1) Identify a producer for the three serotypes of V. cholerae O LPS
a. Analyze the products for structural identity by nuclear magnetic resonance (NMR) and other appropriate methods.
b. Quantify the number of LPS per ml
2) Identify a producer for the monoclonal antibodies against O1 Inaba and O139
a. Select species (rabbit, mouse)
b. Stock hybridoma, sequence V-regions
3) Identify a producer for the reference vaccine
4) Start freeze drying programs for the reference vaccine, LPS preparations and Moab preparations at NIBSC
5) Demonstration that candidate ISs are ‘fit for purpose’ in the in-house LPS inhibition ELISA
6) NIBSC to recruit participants for the collaborative study and distribute reagents
7) Statistical analysis of data sets from participants at NIBSC
8) Write ECBS report, get approval from all participants
9) Submit to WHO-ECBS for approval
10) Peer-reviewed publication of the shortened report
CONCLUSION
WHO ISs for V. cholerae LPS inhibition ELISA will ensure the comparability and quality of in-house assays performed by manufacturers and NRAs and will support assay development. The availability of ISs is crucial to safeguard the supply of effective OCVs to the WHO stockpile and to markets in general.
This meeting showed that manufacturers, regulators and experts are willing to meet and share valuable scientific knowledge and experience for a common good, and that consensus on WHO ISs can be reached within such as diverse group.
FUNDING
The meeting was funded by the Bill & Melinda Gates Foundation (BMGF) (Investment ID: OPP1189336).
ACKNOWLEDGEMENT
We gratefully acknowledge the Bill & Melinda Gates Foundation (BMGF) for the funding of this meeting and the Governments of Sweden (from Sida), Republic of Korea and India for their core support to IVI. We also acknowledge Korea Support Committee for IVI (KSC) and all the public- and private-sector organizations and individuals that support IVI’s research and programs. We thankfully acknowledge all the participants at the meeting for their valuable contribution and commitment. Before submission for publication, all the delegates got the chance to review the paper and provide input.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.







