INTRODUCTION
In developing countries, improvement in the health care has contributed to people living healthier and longer lives. This has resulted in an increase in the world ageing population and consequently an increase in age related disorders like dementia. Dementia is debilitating disease which involves variety of conditions that develop when nerve cells (neurons) in the brain die or do not function properly. Alzheimer’s disease (AD) is accounted to be the most common form of dementia followed by vascular dementia and mixed dementia. The symptoms include memory loss, changes in behaviour, ability to think clearly, eventual impairment of one’s ability to carry out basic bodily functions such as walking and swallowing, and ultimately lead to death. It has been estimated that 35.6 million people were suffering from dementia in 2010 and is projected to triple by 2050. Each year 7.7 million new cases are reported which implies that one new case in four seconds is diagnosed every day.1 This disease constitutes a great burden not only to the patients, but is also devastating for the caregivers and is an enormous social and economic burden to the Society. Pharmaceutical interventions so far have been directed towards amelioration of the symptoms, no drug action stops the progression of the disease.
On the other hand, diabetes is common metabolic disease that is manifested in the form of hyperglycemia and glucose intolerance due to relative insulin deficiency, impaired effectiveness of insulin action, or both.2 Worldwide estimations in 2011 indicate 366 million patients suffering from diabetes and these numbers are proposed to rise to 552 million by 203.3 There are two types of diabetes mellitus; Type1 Diabetes Mellitus (T1DM) and Type 2 Diabetes Mellitus (T2DM), which differ in their etiology and clinical presentation. T1DM is an autoimmune disease in which the insulin producing cells of the pancreas are destroyed, and results in chronic hyperglycemia due to insulin deficiency. In contrast, T2DM is a result of insulin resistance and relative insulin deficiency that is caused due to inadequate response by target tissues like skeletal muscles, adipose tissue and liver, to circulating insulin and is often accompanied by raised insulin levels.
Many recent studies have implicated T2DM as a risk factor for cognitive dysfunction and dementia in the elderly. A positive correlation has been observed between the number of elderly individuals with T2DM and the number of people with diabetes and cognitive dysfunction. Rotterdam study is one of the earliest large scale epidemiological finding which showed that T2DM patients had an increased risk for developing dementia.4 Another study in 2004 demonstrated a 65% increase risk of developing AD in T2DM patients.5 Furthermore, a recent meta-analysis of population-based longitudinal studies in 2012 confirmed the relative risk of AD in subjects with T2DM was 1.46 times higher as compared with the subjects without T2DM.6 Studies have revealed that AD is a metabolic disease, and its symptoms are associated with impairments in brain’s responsiveness to insulin, glucose utilization and energy metabolism, which lead to increased inflammation, oxidative stress and more insulin resistance. These metabolic derangements contribute to the structural, functional, biochemical and molecular abnormalities that result in neuronal loss, synaptic disconnection, beta amyloid accumulation and tau hyperphosphorylation that are characteristic of AD pathology.7 The concept of AD to be a metabolic disease stemmed from the observations that deficits in cerebral glucose utilization, were present either prior to or coincident with initial stages of cognitive dysfunction.8-11 Even post-mortem studies of AD patient brains show molecular and biochemical evidence of insulin and Insulin like growth factor (IGF) resistance and impairments in signal transduction.12,13 Either, the gene expression of insulin or IGF polypeptides in brain and cerebrospinal fluid is altered or there is reduced level of insulin binding to its receptor and decreased responsiveness to insulin stimulation.12-14 Hence, AD related neurodegeneration has been hypothesized to be “Type 3 diabetes” or “brain insulin resistant state”.
Current pharmacological interventions have made remarkable advances to treat and prevent classic microvascular and macrovascular complications in patients with T2DM,15 however cognitive dysfunctions are not targeted. Elderly T2DM patients with cognitive impairment and dementia face major hindrance in self-care behaviour that is essential for the management of diabetes. Therefore, diabetes along with cognitive dysfunction in the elderly creates a large burden for the patients as well as their families and society. There is an urgent need to take brain protection into consideration while developing and implementing future treatments of diabetes for the elderly population. The underlying mechanisms of the association of T2DM with cognitive impairments need to be investigated and alternative disease modifying approaches are necessary for development of a treatment or method of prevention. Moreover, it may be beneficial to control the blood glucose levels to optimum and establish a combination therapy for enhancing cognitive preservation.
Traditional medicinal system has utilized the potential of medicinal plants since antiquity for the treatment of memory dysfunction. In the present study, the efficacy of a combination therapy of metformin and a US patented novel polyherbal formulation, containing Bacopa monnieri, Hippophae rhamnoides, and Dioscorea bulbifera in management of cognitive dysfunction in T2DM patients was investigated. The polyherbal formulation has demonstrated a potential role in treatment and prevention of neurodegenerative disorders in the elderly by significantly improving cognitive and neuropsychiatric measures, reducing oxidative stress and neuroinflammation.16 Whereas, metformin is the first line pharmacologic treatment for T2DM, as it decreases hepatic glucose production and improves insulin sensitivity by augmenting glucose uptake in the peripheral tissues, mainly muscles.17,18 Metformin’s efficacy, security profile, beneficial cardiovascular and metabolic effects and its ability to be associated with other antidiabetic or neuroprotective agents provided the basis to use the combination therapy in the present study. With this background, this study was undertaken to explore the efficacy of the polyherbal drug in combination with metformin to ameliorate cognitive impairments in T2DM patients.
METHODS
Study Design, Participants and Treatment
The study was conducted as a randomized, single blinded, placebo controlled observational study. Elderly persons with diabetes >60 years of age were enrolled in the study for a period of 24 weeks.
Inclusion criteria were:
Onset of T2DM for at least 10 years, fasting blood glucose levels <180 mg/dl, HbA1c level of 7 to 10%, Body Mass Index (BMI) <40 kg/m2, no autoimmune disorder, no cardiac ischemic or renal disease, no chronic inflammatory disease or infection, no regular consumption of other herbal drugs, no consumption of any vitamin supplements <2 months before starting screening. Additional inclusion criteria were deterioration of memory along with at least three of the following five complaints: poor orientation, poor judgment and problem solving difficulties, trouble in the functioning of community affairs, inability to function independently in home and during hobbies and difficulties in personal care.
Exclusion criteria were:
Non adherence to the study protocol (no consumption of more than 20% of the capsules), any sensitivity or unwanted effect to the test drug after the onset of the study, any variation in patients routine treatment i.e., variation in type and dose of the drugs to be consumed, and treatment with insulin, no consumption of any nutritional/ herbal supplements, consumption of alcohol or narcotic drugs.
Around 235 elderly were screened out of which 115 were excluded for not meeting the exclusion criteria and other reasons. Only 120 patients were randomized into two groups of 60 participants each; Group I: elderly subjects with diabetes who were given metformin and placebo, Group II: elderly patients with diabetes who were given metformin and test drug (polyherbal formulation).16 A random list of numbers was determined by a computer-generated series with the proper sequence applied to container labels and supplied to participants in the order enrolled after being randomly assigned to the various treatment groups. This computer generated randomization scheme was developed and kept by the study sponsor. Metformin and test drug were given at a dose of 500 mg twice daily just after meals. The placebo tablets were prepared using dibasic calcium phosphate and microcrystalline cellulose and were identical in appearance to the test drug.16 Follow up of the patients was performed by monitoring them weekly by phone to control them from nonconsumption of capsules, prevent sample loss and record response to relevant questions. Compliance was determined by calculating the rate of tablet consumption before they received medication for next two weeks. The participants were also asked to contact the study centre if they experienced any medical problems during the study period and were advised to not make any changes in their usual diet, and make any self-reliant changes in the medication routine.
Measurements
Demographic characteristics including age, gender, height, weight, marital status, education, occupation, duration of onset of the disease, type and dose of medication used to control diabetes, cognitive impairment symptoms were assessed and recorded by interviewing the patients. Cognitive parameters and biochemical tests were recorded at baseline and at 12th and 24th week of intervention. Body Mass Index (BMI) was calculated by weight (kg) divided by height squared (m). Cognitive function was assessed following structured performance tests, which included mental status, verbal memory, complex psychomotor skills, and attention/executive functions. Mini mental state examination (MMSE) was used to assess mental status.19 Memory scores were tested using the digital memory apparatus (Medicaid systems, Chandigarh, India) device for both immediate and delayed memory performance. Complex psychomotor skill was examined using the Digital Symbol Substitution (DSS) test, which is a sub-test of the Wechsler Adult Intelligence ScaleRevised,20 and has a score range of 0-93. Attention span scores were obtained using the electronic device-Attention Span Apparatus (Medicaid systems, Chandigarh, India). A well-trained psychologist/technical person administered all four tests in the same order to all the study patients. Depression was assessed by the Geriatric Depression Scale-15 (GDS-15),21 which is a global test for depression with scores ranging from 0 to 15. Functional activity questionnaire (FAQ) scores were also obtained to test cognitive function of the participants.22,23 After 12 h of fasting venous blood sample was taken from each patient by a laboratory technician at the beginning, follow-ups and end of intervention. The blood samples were collected in separate vacutainer tubes and were centrifuged at room temperature, at 3500 g for 10 min; the serum and plasma were stored at -80 °C until they were analysed. Blood glucose, HbA1c, serum insulin, and lipid profile which included total cholesterol, triglycerides, Low Density Lipoprotein (LDL), High Density Lipoprotein (HDL) were analysed using standard tests using blood serum. The blood plasma level of homocysteine was determined by a Highperformance liquid chromatography (HPLC) method. Levels of CRP, IL-6 and TNF-α were detected in the blood plasma by the ELISA method using test kits. SOD and GPx activities as well as GSH and TBARS levels were measured as markers for oxidative stress. SOD activity was estimated by the method of Misra and Fridovich,GPx activity was estimated by the method of Rotruck, et al. GSH levels as well as TBARS levels were detected in the blood by colorimetric assays.24-29 Any adverse reactions were assessed by routinely monitoring liver function test (serum glutamic-oxaloacetic transaminase, glutamic pyruvic transaminase, bilirubin and alkaline phosphatase), total protein and serum albumin, urine test (urinary vanillomandelic acid, 17 ketosteroides and glucocorticoid) and kidney function tests (blood urea, serum creatinine, and uric acid) using standard laboratory procedures.
Ethical Considerations
The study aims and methods were explained to the patients and the informed written consent was received from them if they were interested in participation. The study protocol was approved by the institution’s ethical committee at Institute of medical science, Banaras Hindu University and SRM University. The clinical trial titled ‘‘Prevention and management of age related neurodegenerative disorders-an Ayurvedic intervention’’, was registered (No. K.11022/10/2009-DCC) with Dept. of AYUSH (Ministry of Health and Family Welfare, Govt. of India). The study was approved by Institutional ethical committee, and was undertaken as an additional pilot trial under the CTRI/2014/12/005312 trial, to specifically study subjects with diabetes and cognitive impairment.
Data Analysis
All data are expressed as mean±SD. The unpaired student t test was performed to compare the results obtained from the different groups. All statistical analysis was done using the Graphpad prism ver. 2.0. Per-protocol analysis was performed and statistical significance was regarded at P<0.05.
RESULT
Demographic Data of Subjects
A total of 120 elderly subjects with diabetes participated in the study, out of which the 112 participants completed the study. The following cases were excluded from the intervention: 4 patients had no tendency to continue, 3 patients were irregular for weekly follow-ups due to travel, 1 patient could not be contacted; the remaining 112 participants continued till the end of the trial were all investigated (Figure 1). The compliance of consuming capsules in both the groups turned out to be more than 90%, thus demonstrating a well adherence to the study protocol by the patients. All the patients who received the combination therapy, 66(58.9 %) were male and 46(38.3%) were female. The mean age of the patients in group I and II were 65.92±6.87 and 67.48±8.35 respectively. The baseline characteristic of the patients before the study is presented in Table 1. There were no statistically significant differences between the variable in the two groups (Table 1). The adverse events reported in the study were mild in severity and included nausea, drowsiness and constipation. The study parameters after 24 weeks of treatment, including neuropsychological parameters, memory span, biochemical, inflammatory and oxidative stress parameters were compared between group I and II and are shown in Tables 2, 3, 4 and 5 respectively.
Figure 1: Flow chart of the study
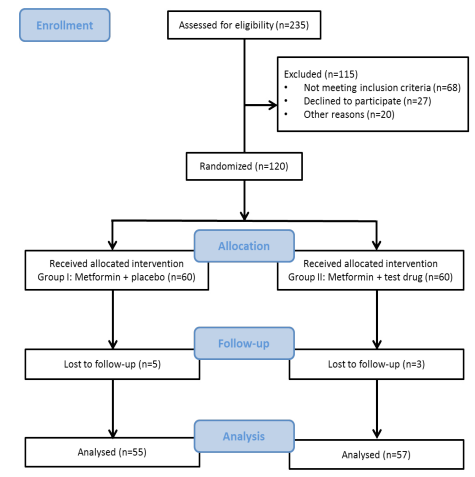
Table 1: Comparison of the qualitative and quantitative variables between the two groups before the intervention.
|
Variables
|
Group I (N=55) Mean+SD |
Group II (N=57) Mean+SD |
P- value |
| Male (N (%)) |
31(56.36) |
35(61.4) |
|
|
Female (N (%))
|
24(43.63) |
22(38.59) |
|
| Age (years) |
65.92±6.87 |
67.48±8.35 |
0.28
|
|
Body mass Index (kg/m2)
|
25.04±6.85 |
26.39±5.27 |
0.24 |
| Blood glucose (mg/dL) |
156.17±43.58 |
164.27±54.91 |
0.39
|
|
HbA1c (%)
|
10.09±2.31 |
9.63±3.24 |
0.39 |
| Serum Insulin (mU/ml) |
8.39±3.48 |
7.85±2.72
|
0.36
|
|
Serum Cholesterol (mg/dL)
|
219.58 ± 41.56 |
228.41±33.75 |
0.22 |
| Serum Triglycerides (mg/dL) |
225.88±62.04 |
234.71±48.90 |
0.40
|
|
Serum LDL (mg/dL)
|
122.42±11.73 |
118.42±14.82 |
0.12 |
| Serum HDL (mg/dL) |
46.45±3.72 |
48.73±5.01 |
0.16
|
|
Homocysteine (Hcy) (nmol/L)
|
21.852±5.921 |
22.52±3.91 |
0.48 |
| C-reactive Protein (CRP) (mg/dL) |
4.853±1.574 |
5.063±1.74 |
0.50
|
|
Interleukin-6 (IL-6) (pg/dL)
|
1.392±0.041 |
1.401±0.026 |
0.17 |
| TNF-α (pg/ml) |
987.26±91.23 |
1010.53±105.21 |
0.21
|
|
MMSE score
|
15.24±2.90 |
15.93±2.16 |
0.56 |
Effect on Neuropsychological Parameters
To assess the therapeutic potential of metformin in combination with test drug for neuroprotective effects in patients with diabetes, several neuropsychological parameters such as memory, mental status, complex psychomotor skills, and attention/executive functions were analysed and are shown in Table 2. After 24 weeks of intervention the neuropsychological parameters were compared in elderly patients with diabetes who received a combination therapy of metformin and placebo or metformin and test drug. Significant improvement in the MMSE score (P<0.0001), DSS score (P<0.0001), word recall (P<0.0001), attention span (P<0.0001), FAQ (P=0.0320), and HDS score (P<0.0001) was observed. Similar statistical analysis on the short term memory (P<0.0001), and long term memory scores (P<0.0001) confirmed significant improvements in group II that received a combination therapy of the test drug with metformin.
Table 2: Effect of combination therapy on neuropsychological parameter scores.
Values are expressed as mean±SD
Group I (N=55) elderly diabetics treated with metformin and placebo, group II (N=57) elderly diabetics treated with metformin and test drug, Statistical analysis have been done to compare scores after 24 weeks treatment between group I vs. II
MMSE: Mini Mental State Examination; DSS: Digital Symbol Substitution; FAQ: Functional Activity Questionnaire; HDS: Hamilton Depression Scale
|
Parameter
|
Group I (N=55) |
Group II (N=57) |
P value (comparison between Group I and II after 24 weeks) |
| |
Initial |
After 12 weeks |
After 24 weeks |
Initial |
After 12 weeks |
After 24 weeks |
|
|
MMSE score
|
15.24±2.90 |
15.12±3.31 |
14.89±2.01 |
15.93±2.16 |
16.28±3.20 |
17.95±3.11 |
<0.0001 |
| DSS score |
41.98±12.49 |
40.80±11.97 |
41.72±12.05 |
39.72±10.91 |
43.85±3.85 |
47.90±13.06 |
<0.0001
|
|
Word recall score
|
4.87±1.22 |
5.06±1.30 |
4.69±2.21 |
5.02±1.83 |
6.42±1.69 |
6.85±2.04 |
<0.0001 |
| Attention Span score |
6.68±2.13 |
6.35±1.87 |
6.49±1.32 |
6.98±1.63 |
7.55±2.04 |
8.09±2.12 |
<0.0001
|
|
FAQ score
|
19.842±4.316 |
18.975±3.922 |
18.895±3.116 |
20.101±5.824 |
18.702±6.102 |
17.186±4.993 |
0.0320 |
| HDS score |
14.867±3.085 |
13.352±4.113 |
12.886±3.591 |
15.854±4.753 |
16.104±4.902 |
17.372±5.112 |
<0.0001
|
Effect on Biochemical and Inflammatory Markers
The levels of inflammatory markers and other biochemical parameters have been reported in Table 3. No significant statistical differences were observed at the beginning of the intervention. However, there was gradual increase in the Hcy levels in group I, suggesting a progressive neurodegeneration due to glucose intolerance. Comparison of Hcy levels between groups I and II at the end of 24 weeks showed significant decrease in Hcy levels (<0.0001). Similar trend of decrease in CRP level (P=0.0003) was observed in the group that received the test formulation in addition to metformin. Additionally, the levels of inflammatory markers IL-6(P<0.000) and TNF-α (P<0.0001) were significantly lowered after 24 weeks of intervention. According to the results, no statistical differences were observed at the beginning and end of intervention in terms of serum insulin (P=0.0525) and HbA1c (P=0.6489) levels.
Table 3: Effect of combination therapy on memory span.
Group I (N=55) elderly diabetics treated with metformin and placebo, group II (N=57) elderly diabetics treated with metformin and test drug, Statistical analysis have been done to compare scores after 24 weeks treatment between group I vs. II.
STM: Short Term Memory; LTM: Long Term Memory
|
Memory span (Score)
|
P value (comparison between Group I and II after 24 weeks)
|
|
|
Group I (N=55) |
Group II (N=57) |
|
| Initial |
After 12 weeks |
After 24 weeks |
Initial |
After 12 weeks |
After 24 weeks
|
|
|
STM score
|
7.84±1.63 |
7.39±1.82 |
7.1±1.58 |
6.99±1.81 |
7.83±1.20 |
8.25±2.04 |
<0.0001 |
| LTM score |
5.98±1.02 |
5.82±0.97 |
5.66±1.22 |
5.61±1.13 |
5.89±1.35 |
6.75±1.38 |
<0.0001
|
Table 4: Effect of combination therapy on inflammatory markers and biochemical parameters.
Values are expressed as mean±SD
Group I (N=55) elderly diabetics treated with metformin and placebo, group II (N=57) elderly diabetics treated with metformin and test drug, Statistical analysis have been done to compare scores after 24 weeks treatment between group I vs. II.
Hcy: Homocysteine; CRP: C-reactive Protein; IL-6: Interleukin 6; TNF- α: Tumor necrosis factor alpha
|
Parameter
|
Group I (N=55) |
Group II (N=57) |
P value (comparison between Group I and II after 24 weeks) |
| |
Initial |
After 12 weeks |
After 12 weeks |
Initial |
After 12 weeks |
After 12 weeks |
|
|
Hcy (nmol/L)
|
21.852±5.921 |
20.956±6.321 |
23.721 ±5.456 |
22.52±3.91 |
20.832±5.391 |
20.832±5.391 |
<0.0001 |
| CRP (mg/dL) |
4.953±1.574 |
4.392±2.014 |
5.012±2.101 |
5.063±1.74 |
4.223±1.735 |
3.851±1.080 |
0.0003
|
|
IL-6 (pg/dl)
|
1.392±0.041 |
1.52±0.058 |
1.428±0.085 |
1.401±0.026 |
1.274±0.093 |
9.54 ±1.75 |
<0.0001 |
| TNF-α (pg/ml) |
987.26±91.23 |
1012.41±89.23 |
992.06±92.2 |
1010.53±105.21 |
889.32±86.35 |
760.25±93.26 |
<0.0001
|
|
Serum Insulin (mU/ml)
|
8.39±3.48 |
6.94±2.85 |
7.08±3.10 |
7.85±2.72 |
6.34 ±2.11 |
6.12±1.98 |
0.0525 |
| HbA1c (%) |
10.09±2.31 |
10.35±1.94 |
9.01±1.58 |
9.63 ±3.24 |
9.54 ±1.75 |
8.89±1.18 |
0.6489
|
Table 5: Effect of combination therapy on oxidative stress markers.
Values are expressed as mean±SD
Group I (N=55) elderly diabetics treated with metformin and placebo, group II (N=57) elderly diabetics treated with metformin and test drug, Statistical analysis have been done to compare scores after 24 weeks treatment between group I vs. II.
SOD: Superoxide dismutase; GPx: Glutathione peroxidase; GSH: Glutathione; TBARS: Thiobarbituric acid reactive substances
|
Parameter
|
Group I (N=55) |
Group II (N=57) |
P value (comparison between Group I and II after 24 weeks) |
| |
Initial |
After
12 weeks |
After
24 weeks |
Initial |
After
12 weeks |
After 24
weeks |
|
|
SOD (U/g Hb)
|
1592.11±106.21 |
1422.31±115.32 |
1342.41±102.98 |
1612.23±196.52 |
1423.32±211.32 |
1226.32±150.26 |
<0.0001 |
| GPx (U/g Hb) |
69.31±10.21 |
68.21±11.25 |
71.58±14.65 |
67.24±9.65 |
61.38±12.985 |
58.96±9.35 |
<0.0001
|
|
GSH (U/g Hb)
|
1.721±0.115 |
1.498±0.098 |
1.352±0.142 |
1.689±0.102 |
2.208±0.206 |
2.65±1.25 |
<0.0001 |
| TBARS (nmol/g Hb) |
152.68±40. 17 |
163.68 ±64.05 |
173.45±56.81 |
158.78±65.421 |
139.81±34.72 |
128.17±29.68 |
<0.0001
|
Effect on Oxidative Stress
The effect of the test drug was also assessed on antioxidants like SOD, GPx, GSH and TBARS. A slight increase in oxidative stress markers, SOD, GPx and TBARS was observed in group I that received metformin and placebo. Whereas, the levels of SOD (P<0.0001), GPx (P<0.0001), GSH (P<0.0001), and TBARS (P<0.0001) showed significant decrease at the end of the intervention between group I and II. This demonstrates the ability of test drug to lower oxidative stress in elderly patients with diabetes.
DISCUSSION
The present study indicated that combination treatment of (polyherbal formulation) in addition to metformin in elderly patients with diabetes for 24 weeks causes improvement in cognitive functions. One of its definitive outcomes is a significant change in MMSE scores as well as in DSS, attention span, word recall, FAQ and HDS scores, thereby showing the effect of the polyherbal formulation in controlling neurodegeneration in T2DM patients. On the other hand, a significant decrease was found in oxidative stress and inflammatory markers in the group that received metformin and test drug compared to the group that received metformin and placebo. Therefore, considering the results of these parameters especially MMSE, inflammation and oxidative stress, it may be concluded that the polyherbal formulation is effective in preventing progressive neurodegeneration associated with diabetes.
Aging is a major risk factor for AD and evidences from recent studies suggest that brain insulin resistance is one of the major factor that contribute to mild cognitive impairment or dementia and AD.12,30-34 The molecular and biochemical consequences of insulin resistance in the brain are caused due to impairments in the insulin signalling pathway that compromises neuronal survival, energy production, gene expression, plasticity and white matter integrity.33,35,36 The brain undergoes a starvation state due to deficit in glucose uptake and utilization, thus causing oxidative stress, impairments in homeostasis and increased cell death. Impairments in insulin signalling results in neurodegeneration due to increased activity of kinases that aberrantly phosphorylates tau, generation of reactive oxygen species that damages proteins, nucleic acids and lipids, leads to accumulation of amyloid beta monomers and plaques, causes mitochondrial dysfunction and increase signalling through pro-inflammatory and pro-apoptosis cascades.35-38 Also insulin resistance is associated with down-regulation of genes needed for cholinergic function, thereby further compromising neuronal plasticity, memory and cognition.36,38
The basis of the neuroprotective effects of the test formulation is corroborated by several studies in animals and humans. The polyherbal formulation is a combination of Bacopa monnieri, Hippophae rhamnoides and Dioscorea bulbifera. Several studies have demonstrated the nootropic effect of Bacopa monnieri is imparted via the action of triterpeniod saponin called bacosides that show acetylcholinesterase inhibition, acetyltransferase activation, b-amyloid reduction, and increased cerebral blood flow.46 Hippophae rhamnoides on the other hand contains high concentration of flavonoids, fat soluble vitamins, folic acid, various fatty acids, phytosterols, essential amino acids and quercetin as the active phytomolecule. The fruit pulp extract Hippophae rhamnoides have a potent antioxidant property that imparts a neuroprotective role by preventing oxidative damages. In addition, high concentration of folic acid in the fruits helps in regulating homocysteine metabolism by lowering the elevated levels associated with neurodegeneration.39,40 Moreover, Dioscorea bulbifera extracts contain diosgenin and studies have shown its potent anti-inflammatory, anti- hyperglycemic and anti-obesity properties.41-44 Its role in preventing neurodegeneration can be postulated to be via management of neuroinflammation caused due to hyperglycaemia, hyperlipoproteinaemia and obesity. After assessing the pharmacological activities of the plant extracts it was concluded that whole plant of Bacopa monnieri, fruit pulp of Hippophae rhamnoides and rhizome of Dioscorea bulbifera possessed AChE inhibitory activity, anti neuroinflammatory and antioxidant properties.45 Several preclinical analysis and clinical trials have proven the efficacy of these plants in management of cognitive deficits in aged population.16,43,46-50
Subsequently, metformin in addition to its antidiabtic properties has been also recently recognized as a potential treatment for neurodegenerative disorders such as AD. Studies have shown that metformin prevents apoptotic cascade in endothelial cell type by inhibiting Permeability Transition Pore (PTP) and blocking the release of cytochrome-c that will lead to cell death.51-53 Another study demonstrated that insulin in addition to metformin activates insulin signalling pathway and potentiates insulin’s effects on amyloid reduction, improves neuronal insulin resistance and glucose uptake.53 Moreover metformin has a role in promoting neurogenesis in rodent and human cultures by activating protein kinase C-CREB binding protein (PKC-CBP) pathway, thereby recruiting neural stem cells that regenerates brain by endogenously repairing the injured areas.54
Since the aging population is increasing at an alarming rate, hence people suffering from this T2DM associated cognitive dysfunction will become increasingly larger problem. The need of the hour is to understand the underlying mechanisms and pathophysiology of this specific condition that may lead to development of better therapeutics. It is essential to control optimal level of blood glucose and explore the best combination of medication for establishing and enhancing cognitive preservation. Also, cognitive dysfunction in T2DM may start at a relatively early stage, thus starting early management may be important to prevent not only dementia but also other complications.55
The present study demonstrates a high percentage of patient compliance which attests the clinical findings of improvement in cognitive functions and can be regarded as strong point of the study. The main drawback of the study is small number of patients and a short follow-up. However, few studies have been done to clinically test combination drugs to manage T2DM and cognitive dysfunction and this study proves the requirement of combination treatment in this specific condition. Therefore, it is suggested that forthcoming similar investigations should be done for longer duration with larger sample size. Moreover, studies are warranted to understand the mechanism of action of the test drug in improving cognitive functions in T2DM patients.
CONCLUSION
The observations from the present study indicate that a combination therapy of a polyherbal formulation in addition to metformin for 24 weeks has a potential to be a new therapeutic for patients suffering from cognitive impairments associated with T2DM. Results show a significant improvement in neuropsychological parameters including learning and memory functions in the group that received polyherbal formulation. The study also suggests these changes occur due to neuroprotective effects of the polyherbal formulation by reducing oxidative stress and inflammation. Further, analysis is needed to confirm routine use of this combination therapy.
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interests.
ACKNOWLEDGMENT
We thankfully acknowledge the financial assistance provided by the Department of Ayush and University Grants Commission, Government of India, New Delhi. The study was supported by the project titled “Prevention and management of age related neurodegenerative disorders- an Ayurvedic intervention” (No. K.11022/10/2009-DCC) by Dept. of AYUSH (Ministry of health and family welfare, Govt. of India). The study was approved by Institutional ethical committee, and was undertaken as an additional pilot trial under the CTRI/2014/12/005312 trial, to specifically study subjects with diabetes and cognitive impairment. Additional support was provided by SRM University, Chennai. Thanks to the clinical investigators Prof. V N Mishra and Prof. Malvin George for their help and support in conducting the clinical trial. The study was conducted under the supervision of Prof. G.P. Dubey (Life Long Distinguished Professor, BHU; Study Director & Coordinator, Collaborative program, IMS, BHU. He is the guarantor and takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript. No competing financial interests exist for any authors. Finally we would like to thank the participants and their families for taking part in this study.
AUTHOR CONTRIBUTIONS
G.P.D is the study co-ordinator who conceived and designed the study protocol, he had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. A.S drafted the manuscript, and both A.S and P.K.S contributed to the interpretation of findings, and preparation of the final manuscript. P.U and S.S performed the literature search and statistical analysis, contributed to the data collection with supervision from G.P.D., A.A, K.I and V.N.M, who together developed and designed the study and oversaw the data acquisition and analysis. No potential conflicts of interest relevant to this article are present.






