INTRODUCTION
The advent of coronavirus disease-2019 (COVID-19), in March 2020, has forced the entire world into continuously changing dimensions, the emergence of variants has put forward a challenge to scientists and doctors, indeed the entire world to cope with it. The entire world experienced havoc and a death toll due to the coronavirus that led to the emergence of various vaccine platforms developed against changing variants of coronavirus.1
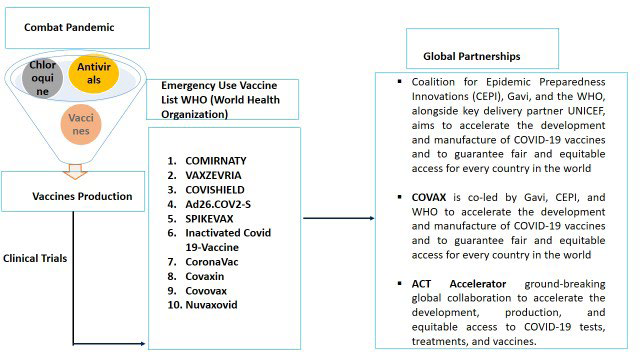
This mini-review highlights how vaccine companies are at the forefront in larger-scale vaccine production at a faster pace synchronizing the clinical trials landscape and global partnerships (Figure 1).
Figure 1. Combat Pandemic: Vaccine Production, Clinical Trials, and Global Partnerships
VACCINE PRODUCTION
India is at the forefront of vaccine production known for its vaccine manufacturing capacity, acclaimed as a vaccine hub accounting for 60% of the global vaccine supply, and a capacity to manufacture over 3 billion COVID-19 vaccine doses per annum.2 Various clinical research studies led to the conclusion that vaccine development was the most viable option to face the pandemic.3 So far, the World Health Organization (WHO), has approved a total of ten vaccines, which include the following types of vaccines.4
Inactivated Vaccines
Inactivated vaccines are formulated by inactivating virulent particles of viruses by treating the virus particle with chemicals. As virus particles are inactivated, they cannot multiply and hence are safer. The limitation of inactivated vaccines is that they have to be introduced in large amounts compared to live attenuated vaccines.4
Viral Vector Vaccines
Adenoviral vectors viral vectors were classically in use to develop vaccines. Their utility depends on their ability to infect cells. The advantage of this platform is its efficient and gene-specific delivery and its initiation of a healthy immune response.4
Messenger Ribonucleic Acid Vaccines
Messenger Ribonucleic acid (mRNA) vaccine comprises elements essential for the encoded protein to be expressed, and most of the time 1-methyl-pseudouridine modification is incorporated in mRNA molecules, to enhance mRNA translation in the body.4 mRNA vaccines were proven superior as they can act as an immunogen, and adjuvant has immunostimulatory characteristics and can stimulate innate immunity without severe side-effects.3
Protein Subunit Vaccines
Subunit vaccines are produced based on synthetic peptides or recombinant technology and are considered a safer and indeed a more reliable method. Various subunit vaccines were approved by Food and Drug Administration (FDA), such as human papillomavirus (HPV), hepatitis B virus, and influenza virus vaccines. Due to fewer side effects and the non-infectious nature of subunit vaccines in comparison to other vaccine types subunit vaccines can be used even in immunocompromised individuals. Limitations include slow manufacture, low immunogenicity, need for cold chain transfer and storage, and adjuvants in clinical trials.5 Nanotechnology-based peptide vaccines have also played a vital role in vaccine development (Figure 2).5
Figure 2. Types of COVID-19 Vaccine Developed Based on Different Technologies1
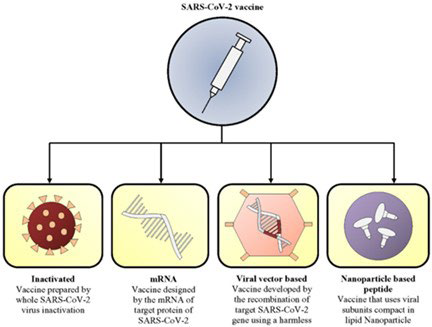
A review was conducted utilizing online databases, (Google Scholar, PubMed, and ScienceDirect) with 59 studies, that have compared different vaccine types (mRNA-based, recombinant, and nanoparticle-based vaccines) are being developed and launched in terms of their efficacy, side effects, and seroconversion based on data reported in the literature and concluded that mRNA vaccines were superior in efficacy, and inactivated ones were with fewer side effects and similar seroconversion was noted in all types of vaccines.1 Approved covid vaccines by WHO were shown in the Table 1.4
| Table 1. WHO Approved COVID-19 Vaccines6 |
|
WHO Approved COVD-19 Vaccines
|
WHO EUL Holder
|
| COMIRNATY® (nucleoside modified) mRNA Vaccine |
BioNTech Manufacturing GmbH |
| VAXZEVRIA (recombinant) Adenoviral ChAdOx1-S |
AstraZeneca AB, AstraZeneca AB/SK Bioscience Co. Ltd |
| COVISHIELD™ (recombinant) Adenoviral ChAdOx1-S |
Serum Institute of India Pvt. Ltd |
| Ad26.COV2-S (recombinant) Adenovira |
Janssen-Cilag International NV |
| SPIKEVAX (nucleoside modified) mRNA vaccine |
Moderna Biotech |
| Inactivated COVID-19 vaccine (Vero Cell) |
ModernaTX, Inc |
| CoronaVac (Vero Cell), Inactivated) |
Sinovac Life Sciences Co., Ltd |
| COVAXIN® (Inactivated Whole Virion) |
Bharath Biotech International Ltd |
| COVOVAX™ SARS-CoV-2 rS Protein Nanoparticle [Recombinant]) |
Serum Institute of India Pvt. Ltd |
| NUVAXOVID™ (SARS-CoV-2 rS [Recombinant, adjuvanted]) |
Novavax CZ a.s |
CLINICAL TRIALS
To address the unmet need of the hour due to the covid pandemic, the WHO has approved the emergency use of 10 COVID vaccines.4 Clinical trials play a pivotal role to assess the immunogenicity, safety, and efficacy of the vaccines for human use.7 In addition to India, the pace at which clinical trials were done in various other countries is noteworthy.
Covaxin®
- Several Phase I (NCT04471519) and II, trials were conducted8
- Phase III trial included 25,800 subjects and was registered on the Indian Clinical Trials Registry India, CTRI/2020/11/028976, and ClinicalTrials.gov, NCT046414819,10
- Vaccine efficacy was found to be 77.8%7
- Clinical trials were conducted on a larger global scale at 100 clinical sites sparing in 20 different countries across the globe.7,10
Covishield™
- Four (4) Randomized, blinded, controlled clinical trials were in progress with covishield (pooled N=23,745).
- Interim analysis of pooled data from 4 clinical trials has shown 70.4% vaccine efficacy at 95.84% CI. • Phase I/II Study, COV001 (NCT04324606), UK; COV005 (NCT04444674) in South Africa a Phase II/III in the UK and in Brazil.9
Covovax™
- Study 1 novel coronavirus-301 (2019nCoV-301) an ongoing study in the US and Mexico and a part of the study also includes, the pediatric expansion study.
- Study 2 (2019nCoV-302) Phase III, the study involves 14,039 participants conducted at the UK and has shown 89.7% efficacy at 95% CI.10
- Phase III (B.1.1.7 variant. Funded by Novavax COVID-19 vaccine2373 (NVX-CoV2373), N=1403) a randomized, observer-blinded, placebo-controlled trial has been conducted at 33 sites in the UK.11,12
Corbevax™
- In phase I/II clinical study (BECT062) conducted on 360 subjects, in order to assess the safety, reactogenicity, and immunogenicity and select the optimum formulation of BE’s severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) (COVID-19), all four formulations were found to be safe and well-tolerated.
- In phase II/III Clinical study (BECT069) was conducted on 1268 subjects.
- Approved pediatric vaccine in India.13,14
Sputnik V
- Clinical trials Phase I/II in Russia (NCT04436471, N=38), Phase III Clinical Trial in Russia (RESIST, NCT04530396, were conducted in 33,771 volunteers.
- In India phase II/III adaptive study (NCT04640233, phase II part on Indian study, N=100, phase III enrolled 1500 Indian subjects), efficacy of the vaccine is 85.6-95.2% at 95 % CI.15,16
ZyCoV-D®
- Single-centre, open-label, non-randomized, Phase 1 trial in India (N=126, CTRI/2020/07/026352).
- Vaccine was found to be safe, well-tolerated, and immunogenic.17
GENNOVA BIOPHARMA
- Department of Biotechnology-Biotechnology Industry Research Assistance Council (DBT-BIRAC) has facilitated the establishment of a ‘first-of-its-kind’ mRNA-based vaccine manufacturing platform in India and DBT has provided seed funding for the development of Gennova’s novel self-amplifying mRNA-based vaccine candidate for COVID-19 in rodents and primates.18
- Currently, dose-range, placebo-controlled, Phase I study in healthy adult subjects N=72, CTRI/2021/04/032688.19
These efficient clinical trials led to the approval of various COVID-19 vaccines in India and were shown in the Table 2.
| Table 2. Vaccines Produced in India |
|
Company Name
|
Vaccine Type
|
Vaccines Produced
|
| Bharath Biotech |
Inactivated whole virion, BBV152 |
Covaxin |
| Serum Institute
of India |
Adenoviral vector ChAdOx1
SARS-CoV-2 rS Nanoparticle |
Covishield
Covovax |
| Biological E Ltd. |
Protein subunit |
Corbevax |
| Dr. Reddy’s |
Adenoviral Ad25, Ad26 |
Sputnik (GamCovidVac) |
| Zydus Cadila |
Deoxyribonucleic acid (DNA) |
ZyCoV-D® |
| Gennova Biopharma |
mRNA |
HGCO19 |
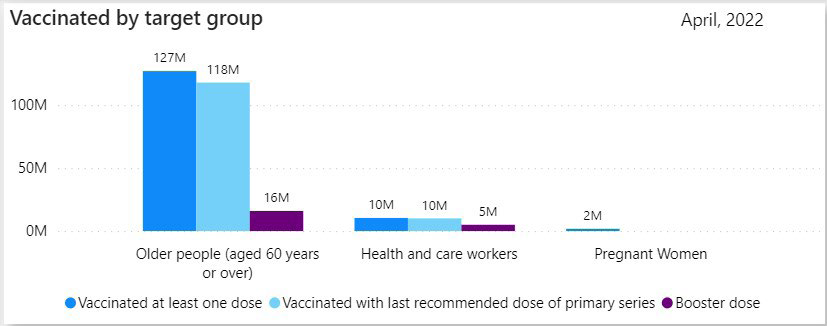
As shown in the above table in India, in pandemic times vaccines of various types were produced.8-15 Management of clinical trials at a faster pace on a larger scale, globally, paved the way to vaccinate a larger population. As of the 19th of May, 1,91,70,35,690 vaccine doses have been administered in India.20 WHO has approved 3 Indian vaccines Covavax, Covaxin, and Sputnik (GamCovidVac). Millions of people have been vaccinated till now as shown in the Figure 3. Vaccines have proven to be vital to combating this Pandemic worldwide.
Figure 3. Graph Depicting People Vaccinated with Primary and Booster Doses by the Target Group till April 2022 (WHO)21
GLOBAL PARTNERSHIPS
In lieu, to bring the pandemic to an end, various global partnerships have emerged,
- Coalition for Epidemic Preparedness Innovations (CEPI), Gavi, the Vaccine Alliance, and the World Health Organization (WHO), alongside key delivery partner United Nations Children’s Fund (UNICEF), aim to accelerate the development and manufacture of COVID-19 vaccines, and to guarantee fair and equitable access for every country in the world. CEPI will provide funding of up to US $19.3 million to support the development of a ‘variant-proof ’ SARS-CoV-2 vaccine candidate to an international multidisciplinary consortium comprising Bharat Biotech International Ltd (BBIL), India, the University of Sydney, Australia, and ExcellGene SA, Switzerland. CEPI has announced another partnership with India, a consortium comprised of the Translational Health Science and Technology Institute (THSTI), an autonomous institute of the DBT, Ministry of Science and Technology, Government of India, and Panacea Biotec, a research-based biopharmaceutical company and vaccine manufacturer, to develop vaccine candidates that could provide broad protection against SARS-Cov-2 variants and other Beta coronaviruses.22 Recently, CEPI has launched an R&D focused three-point agenda to
- Reduce vaccine development timelines to 100, instead of 300- days.
- Develop a universal vaccine against coronaviruses and
- Develop a library of vaccine candidates against other diseases.23
This “moon-shot” goal to produce vaccines in 100-days has been widely adopted by governments throughout the world, and indeed, several vaccine developers are exploring strategies to achieve this aim.24
- The ACT Accelerator is a ground-breaking global collaboration to accelerate the development, production, and equitable access to COVID-19 tests, treatments, and vaccines. WHO launched a COVID-19 Technology Access Pool and called to action all pharmaceutical companies and technology holders to share COVID-19-related knowledge and intellectual property (IP) to accelerate the development of tools to end the pandemic.25,26
- COVAX is co-led by Gavi, CEPI, and WHO. Its aim is to accelerate the development and manufacture of COVID-19 vaccines and to guarantee fair and equitable access for every country in the world. Access to the vaccine for low-income countries (LICs) under the COVAX program and government efforts will play an essential role to achieve global immunity to stop the transmission of the virus.16
- Vaccine Maitri India, has launched a diplomatic mission to supply vaccines (COVAXIN® and COVISHIELD™) to the countries in need. Under this initiative, it has sent vaccine supplies to many countries in the Asia Pacific region.24 In addition to this, under the COVAX, a global initiative, 18.1 million vaccine doses (COVISHIELD™), have been already supplied to various countries.27
- COVID-19 pandemic has resulted in the havoc and the disruption of normal lives, loss of jobs, and loss of trade, and has dwindled the already weak national economies in the LICs.26 The global dashboard for vaccine equity is a joint initiative of United Nations Development Programme (UNDP), WHO, and the University of Oxford with cooperation across the e United Nations (UN) system, anchored in the Sustainable Development Goals (SDG 3) Global Action Plan for Healthy Lives and well-being for all.28 It shows that the vaccine access in high-income countries, 3 in 4 people or 72.09% have been vaccinated with at least one dose whereas, in low-income countries, 1 in 6 people, or 17.92% have been vaccinated with at least one dose as of May 25, 2022.28 In the current scenario, equitable access to vaccines especially for the front-line workers is critical to mitigating and maintaining public health systems and economic growth.29
CONCLUSION
In changing dimensions of a pandemic, harnessing, collaborations and partnerships, various regulatory landscapes, and the advent of new vaccine technology platforms revolutionized the vaccine development pace, and management of clinical trials effectively at a larger scale led to the development of safe and efficient vaccines, which were proven to be efficient against COVID-19. The emergence of global partnerships emphasizes pandemic preparedness, and vaccine equity to all, which in turn renders global immunity and health, as a right of everyone. India, being a giant vaccine manufacturer, has leveraged, the COVID-19 vaccine, to various countries as commercial exports, and under the COVAX initiative, it is at the forefront in the contribution, of cost-effective vaccines to the world. Insights of global leaders, Industries, Academics, philanthropists, and governments address pandemics in a holistic way, paving a way to combat COVID-19 indeed these pandemic lessons will equip us to cope with, utilize, share and improve our resources, and tools to manage future pandemic threats (Figure 4).
Figure 4. Combat Pandemic: COVID-19 Vaccines Production, Clinical Trials, and Global Partnerships