INTRODUCTION
Collecting ducts carcinoma or bellini duct carcinoma (CDC) is one of the most uncommon variants of renal carcinomas.1 During last decade, no more than 20 cases have been reported and published in the United States (USA),2,3 and a literature review identified 270 cases over the past 20-years.4 Recently, Sui et al5 published a report of 577 patients which represents the largest cohort of CDC in the literature to date CDC demonstrates highly aggressive behavior, being the variant of renal carcinoma with the worst prognosis and the lowest cancer-specific survival rate,6 as 70% of deaths are secondary to the disease.
Early diagnosis is the main prognostic factor. However, most cases present with distant disease at the time of diagnosis.7 Surgical treatment leads to the highest survival rates, especially small or confined (pT1) tumors. Different treatment protocols have been published, including chemotherapy, radiotherapy and multi-modality therapy, but without favorable responses in the majority of patients.8
The objective of this study is to report a case of CDC which presented with paraneoplastic syndrome, which was surgically treated and required systemic adjuvant treatment. In addition, a literature review of this disease also provided.
CASE REPORT
A 59-year-old male patient with past history of renal lithiasis and hyperuricemia presented with new onset asthenia and adynamia accompanied by night sweats, fever (<37.9 °C) and unintentional weight loss of 8-10 kg over 30-days. He denied abdominal pain, lower urinary tract symptoms, cough or other symptoms.
Physical examination was essentially unremarkable except for pale mucous membranes. Serum profile showed anemia, high leukocyte, neutrophil and platelet count, and increased acute phase reactants. Hematocrit 23.8%, Hemoglobin 7.4 gr/dL, leukocytes: 21.700 ml/mm3, Platelets: 700,000 ml/mm3, Erythrocyte sedimentation rate (ESR) 9 mm, C-Reactive Protein 129 mg/L, Glucose 132 mg/dL, Urea 32 mg/dL, Ionogram (sodium/potassium/chloride): 130 mEq/L / 4.2 mEq/L / 95 mEq/L, alkaline phosphatase level (ALP) 248 IU/L, Creatinine 1.12 mg/dL.
Urinary sediment, human immunodeficiency virus infection (HIV), blood and urine culture were unremarkable. Ultrasound and abdominal computerized tomography (CT) scan (Figures 1 and 2) revealed a 12×5 cm solid mass with heterogeneous enhancing soft tissue density, involving the left renal perihilar region and a accompanying left renal vein invasion with hilar compression level.
Figure 1. Abdominal and Pelvic CT (Coronal Reconstruction). Tumor Lesion of 12 cm in Diameter at Cephalocaudal Level and
5 cm in the Axial Plane. Invasion of the Renal Vein (Direct Invasion and Proximal Stenosis in Topography of the Renal Hilum)
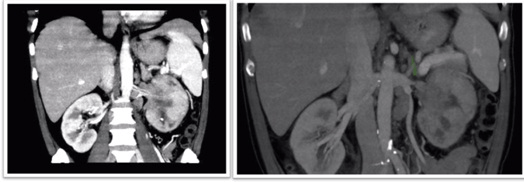
Figure 2. CT Scan (Axial View): Intimate Contact with the Quadratus Lumborum, Muscle and to a Lesser Extent with
the Upper 1/3 of the Illopsoas Muscle. Left Paraaortic and Perihiliar Lymph Nodes
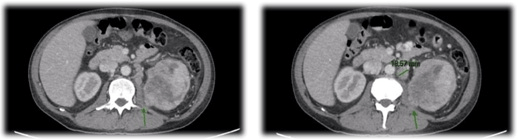
The mass was observed to extend posteriorly to contact the quadratus lumborum muscle and upper 1/3 of psoas, without a clear plane of separation. Multiple left perihilar and para-aortic lymph nodes were visualized (some greater than 2 cm). Renal morphometry score=12 p.
The clinical picture was interpreted as paraneoplastic syndrome secondary to renal neoplasia. Transfusions with red blood cells were indicated following invasive treatment. A coaxial-trucut (17 G coaxial needle and 18 G trucut sheath) biopsy was performed showing infiltration by undifferentiated fusocellar and pleomorphic neoplasia. Immunohistochemistry stained positive for renal cell carcinoma (RCC), cytokeratin (CK)7, CD10 binding, with biphasic phenotype consistent with sarcomatoid carcinoma.
The patient opted for surgical treatment which was performed via xiphoid-pubic midline incision. Radical nephrectomy with left para-aortic lymphadenectomy was performed with dissection of the retroperitoneum from the left iliac bifurcation to the left diaphragmatic pillar including the proximal and mid-left ureter. The post-operative course was uneventful, with hospital discharge on post-operative day (POD) number.4
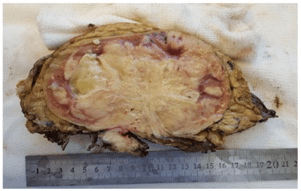
On gross pathologic analysis, a grayish white tissue tumor measuring 12.5×9 cm was described. The tumor invaded the renal sinus and hilarfat, but did not extend through Gerota’s fascia (Figure 3).
Figure 3. Macroscopy: Renal Mass (12.5×9 cm in Diameter)
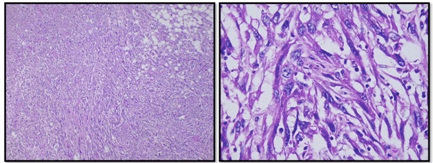
Microscopy (Figure 4) revealedun differentiated sarcomatoid carcinoma (90%) with 50% necrosis, Fuhrman grade 4. Margins were negative. Lymphadenectomy showed eleven normal lymph nodes, thuspathological stage: pT3a N0.
Figure 4. Microscopy: Carcinoma with a Sarcomatoid Phenotype (Spindle Cell)
Immunohistochemistry shown in Figures 5 and 6, also stained positive for Vimentin CD 10, CK7 and CK19, and negative for p53. CDC with sarcomatoid features was the final histologic diagnosis.
Figure 5. Inmunohistochemistry (IHC): CD10 – positive and CD7 – positive
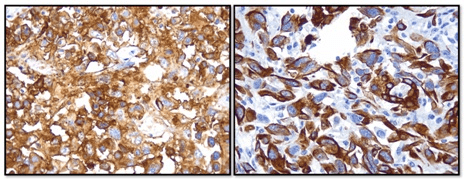
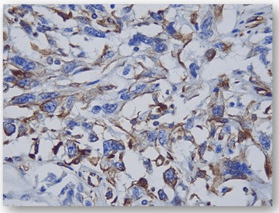
Figure 6. IHC: Vimentin Positive
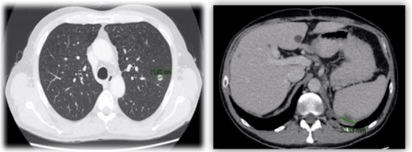
During the fourth month of follow-up, a chest and abdomen CT scan showed a 12 mm nodular lesion in left superior pulmonary lobe and a 31 mm lesion in upper pole of the spleen just below the diaphragm, consistent with metastases (Figure 7).
Figure 7. Chest/Abdomen/Pelvis CT Scan (Following Initial Surgical Treatment): Local Recurrence and Lung Metastasis
A biopsy of the sub-diaphragmatic lesion was performed using a coaxial-trucut system showed recurrent fusocellular and epitheloid neoplasia, with extensive necrosis.
The patient is now receiving a second cycle of adjuvant chemotherapy (Cisplatin-Gemcitabine) without significant adverse events.
DISCUSSION
In 1976, Mancilla-Jiménez et al published the first CDC case, which reported the hyperplastic and atypical changes adjacent to epithelium of collecting ducts in 3 of 34 cases of renal carcinoma.9 The embryological origin differs from other renal parenchymal tumors. The renal collecting system (ureters, pelvis, calices and collecting ducts) are derived from the ureteric bud, which originates from the Wolffian duct, while the renal parenchyma derives from the metanephric blastema. This explains the different clinical, radiological, macroscopic, microscopic, immunohistochemical and cytogenetic characteristics of CDC vs RCC.10
According to the conference of International Society of Urological Pathology (ISUP) on renal neoplasia in Vancouver in 2013, CDC must include at least some medullary lesions, have a predominant tubule formation, and have an inflamed desmoplastic stroma. They must have high-grade cytological features, infiltrative growth patterns, and lastly, no features compatible with other subtypes of renal carcinoma or urothelial carcinoma.11 Immunohistochemistry, it is usually positive for high molecular weight cytokeratins (CK19, CK7, CK8/CK18), Fez1, mucin, lysozyme and lectins.12
Tokuda et al published the largest series and reported an incidence 0.4 to 1.8% of all renal tumors, with 70% prevalence in male and young adults (mean 58.2-years).4
More than a half of patients (65.4%) are symptomatic at the time of diagnosis. The most common presenting symptoms are hematuria and lumbar or abdominal pain, but patients with systemic symptoms such as fever of unknown origin, weight loss, or elevated acute phase reactants are not uncommon. Patients with systemic (paraneoplastic) symptoms often have advanced or distant disease at diagnosis when compared with other types of renal carcinomas.13 More than 75% of CDC present with pT2-pT3 disease with 95% of nuclear grade.4
The most common sites of metastasis are regional lymph nodes, lung and bone. In the Tokuda et al series, 44.2% of patients presented lymphatic metastasis and 32.1% distant metastases.
During the pre-operative evaluation, CT scans are not specific so there is difficulty differentiating from other subtypes of renal carcinomas. In some cases, reporting the use of angiography, CDC is typically hypovascular, while 90% of clear cell renal carcinomas are hypervascular.14
Prior reports observe cancer specific survival, of 69% at one year post diagnosis, falling to 45% and 34% in third and fifth-year respectively.4 Ciszwesky et al4 postulated that mean time of local recurrence and distant metastasis after nephrectomy was 4.9 and 8.1-months respectively.8
Attempts to control the disease with immunotherapy or chemotherapy have met with limited success. Chemotherapy for urothelial carcinomas is used because of mesonephric origin of CDC. The most common regimen employed has been methotrexate, vinblastine, doxorubicin and cisplatin.4 However, the largest prospective series of 23 patients treated with gemcitabine,15 demonstrate an overall response rate of 26% in metastatic CDC. Oudard et al15 propose gemcitabine as first line treatment in stage IV CDC. In this phase II, multi-center trial, a combination of gemcitabine and cisplatin/carboplatin was utilized in 23 patients with metastatic CDC.15 Overall survival at 1-year was 48%, decreasing to 17% at 18-months. Tokuda, et al studied 34 patients treated with immunotherapy (interferon alpha and gamma and interleukin-2 regimen) and found no response.4
A retrospective review of 64 cases of metastatic, non–clear cell renal cell carcinoma reported on 26 cases of CDC. Of these patients, one had a 5-month partial response to gemcitabine plus cisplatin.16
Currently, Siu et al5 reported 577 CDC patients with overall survival for the metastatic CDC cohort of 6.4-months. On sub-analysis, the utilization of surgery with chemo/radiation was associated with decreased risk of death (HR=0.51, 95% CI: 0.32-0.79) compared to surgery, alone, and also compared with chemo/radiation alone (HR=0.57, 95% CI: 0.37-0.89).
CONCLUSION
The collecting duct carcinoma of Bellini is an uncommon malignant renal neoplasm variant which has a poor prognosis, due to high rates of distant disease at the time of presentation. In this case report, the typical biological behavior of local recurrence and metastasis within a few months after surgery was observed. Due to the rare nature and unfavorable outcomes in CDC, a cooperative group trial to assess possible neoadjuvant and adjuvant chemotherapy prior to and after surgical resection, should be considered.
DECLARATION
This case report is ethically approval by Institutional Ethical Committee.
CONSENT
The authors have received written informed consent from the patient.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.












