1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019; 69(1): 7-34. doi: 10.3322/caac.21551
2. American Cancer Society. Cancer Facts & Figures 2019. Website. https://www.cancer.org/research/cancer-facts-statistics/allcancer-facts-figures/cancer-facts-figures-2019.html. Accessed October 14, 2019.
3. Taketo MM. Reflections on the spread of metastasis to cancer prevention. Cancer Prev Res (Phila). 2011; 4(3): 324-328. doi: 10.1158/1940-6207.CAPR-11-0046
4. Kowalik A, Kowalewska M, Góźdź S. Current approaches for avoiding the limitations of circulating tumor cells detection methods—implications for diagnosis and treatment of patients with solid tumors. Transl Res. 2017; 185: 58-84.e15. doi: 10.1016/j. trsl.2017.04.002
5. National Cancer Institute. SEER Cancer Statistics Review, 1975- 2015. Website. https://seer.cancer.gov/archive/csr/1975_2015/ index.html#contents. Accessed October 14, 2019.
6. Budd GT, Cristofanilli M, Ellis MJ, et al. Circulating tumor cells versus imaging–predicting overall survival in metastatic breast cancer. Clin Cancer Res. 2006; 12(21): 6403-6409. doi: 10.1158/1078- 0432.CCR-05-1769
7. Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Eng J Med. 2004; 351(8): 781-791. doi: 10.1056/NEJMoa040766
8. Cristofanilli M, Hayes DF, Budd GT, et al. Circulating tumor cells: A novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005; 23(7): 1420-1430. doi: 10.1200/ JCO.2005.08.140
9. Hayes DF, Cristofanilli M, Budd GT, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006; 12(14): 4218-4224. doi: 10.1158/1078-0432. CCR-05-2821
10. Rack B, Schindlbeck C, Jückstock J, et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst. 2014; 106(5): pii: dju066. doi: 10.1093/ jnci/dju066
11. Serrano MJ, Rovira PS, Martínez-Zubiaurre I, Rodriguez MD, Fernández M, Lorente JA. Dynamics of circulating tumor cells in early breast cancer under neoadjuvant therapy. Exp Ther Med. 2012; 4(1): 43-48. doi: 10.3892/etm.2012.540
12. American College of Surgeons (ACS). AJCC Cancer Staging Manual. 8th ed: NY, USA: Springer International Publishing; 2017.
13. Giuliano AE, Connolly JL, Edge SB, et al.Ca Breast Cancer— Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017; 67(4): 290- 303. doi: 10.3322/caac.21393
14. Thiele JA, Bethel K, Králíčková M, Kuhn P. Circulating tumor cells: Fluid surrogates of solid tumors. Annu Rev Pathol. 2017; 12(1): 419-447. doi: 10.1146/annurev-pathol-052016-100256
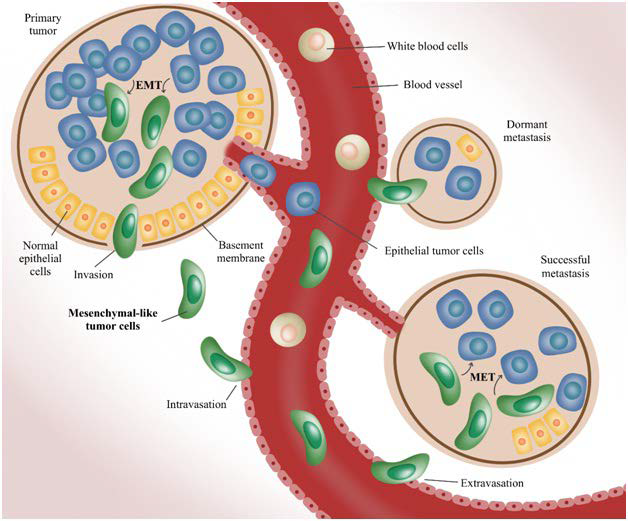
15. Wan L, Pantel K, Kang Y. Tumor metastasis: Moving new biological insights into the clinic. Nat Med. 2013;19:1450-1460. doi: 10.1038/nm.3391
16. Jacob K, Sollier C, Jabado N. Circulating tumor cells: Detection, molecular profiling and future prospects. Expert Rev Proteomics. 2007; 4(6): 741-756. doi: 10.1586/14789450.4.6.741
17. Valastyan S, Weinberg RA. Tumor metastasis: Molecular insights and evolving paradigms. Cell. 2011; 147(2): 275-292. doi: 10.1016/j.cell.2011.09.024
18. Cohen SJ, Punt CJA, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008; 26(19): 3213-3221. doi: 10.1200/JCO.2007.15.8923
19. Danila DC, Heller G, Gignac GA, et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res. 2007; 13(23): 7053-7058. doi: 10.1158/1078- 0432.CCR-07-1506
20. Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008; 359(4): 366-377. doi: 10.1056/NEJMoa0800668
21. Moreno JG, O’Hara SM, Gross S, et al. Changes in circulating carcinoma cells in patients with metastatic prostate cancer correlate with disease status. Urology. 2001; 58(3): 386-392. doi: 10.1016/s0090-4295(01)01191-8
22. Uen Y-H, Lu C-Y, Tsai H-L, et al. Persistent presence of postoperative circulating tumor cells is a poor prognostic factor for patients with stage I-III colorectal cancer after curative resection. Ann Surg Oncol. 2008; 15(8): 2120-2128. doi: 10.1245/s10434-008- 9961-7
23. King JD, Casavant BP, Lang JM. Rapid translation of circulating tumor cell biomarkers into clinical practice: Technology development, clinical needs and regulatory requirements. Lab Chip. 2014; 14(1): 24-31. doi: 10.1039/c3lc50741f
24. de Bruin EC, McGranahan N, Mitter R, et al. Spatial and temporal diversity in genomic instability processes defines lung cancer evolution. Science. 2014; 346(6206): 251-256. doi: 10.1126/science.1253462
25. Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012; 366(10): 883-892. doi: 10.1056/NEJMoa1113205
26. Navin N, Kendall J, Troge J, et al. Tumour evolution inferred by single-cell sequencing. Nature. 2011; 472(7341): 90-94. doi: 10.1038/nature09807
27. Pantel K, Speicher MR. The biology of circulating tumor cells. Oncogene. 2016; 35(10): 1216-1224. doi: 10.1038/onc.2015.192
28. Tsao SC-H, Wang J, Wang Y, Behren A, Cebon J, Trau M. Characterising the phenotypic evolution of circulating tumour cells during treatment. Nat Commun. 2018; 9(1): 1482. doi: 10.1038/s41467- 018-03725-8
29. Paterlini-Bréchot P. Organ-specific markers in circulating tumor cell screening: An early indicator of metastasis-capable malignancy. Future Oncol. 2011; 7(7): 849-871. doi: 10.2217/fon.11.32
30. Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004; 10(20): 6897-6904. doi: 10.1158/1078-0432.CCR-04-0378
31. Politaki E, Agelaki S, Apostolaki S, et al. A comparison of Three methods for the detection of circulating tumor cells in patients with early and metastatic breast cancer. Cell Physiol Biochem. 2017; 44(2): 594-606. doi: 10.1159/000485115
32. Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009; 9(4): 302-312. doi: 10.1038/nrc2627
33. Li J, Sharkey CC, Wun B, Liesveld JL, King MR. Genetic engineering of platelets to neutralize circulating tumor cells. J Control Release. 2016; 228: 38-47. doi: 10.1016/j.jconrel.2016.02.036
34. Cross SE, Jin Y-S, Rao J, Gimzewski JK. Nanomechanical analysis of cells from cancer patients. Nat Nanotechnol. 2007; 2(12): 780-783. doi: 10.1038/nnano.2007.388
35. Shaw Bagnall J, Byun S, Begum S, et al. Deformability of tumor cells versus blood cells. Sci. Rep. 2015; 5: 18542. doi: 10.1038/ srep18542
36. Guck J, Schinkinger S, Lincoln B, et al. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys J. 2005; 88(5): 3689-3698. doi: 10.1529/biophysj.104.045476
37. Farace F, Massard C, Vimond N, et al. A direct comparison of CellSearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. Br J Cancer. 2011; 105: 847-853. doi: 10.1038/bjc.2011.294
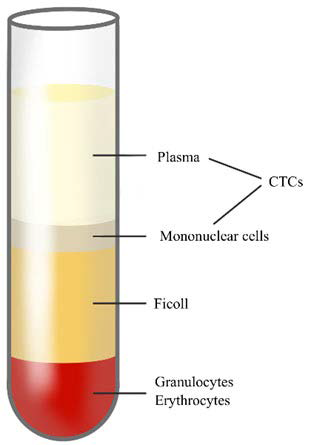
38. GE Healthcare. Isolation of mononuclear cells: Methodology and applications. Website. https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma-Aldrich/General_Information/1/ge-isolation-of-mononuclear-cells.pdf. Accessed October 14, 2019.
39. Gertler RR, Fuehrer K, Dahm M, Nekarda H, Siewert JR. Detection of circulating tumor cells in blood using an optimized density gradient centrifugation. In: Allgayer HMM, Schildberg FW, eds. Staging of Cancer. Recent Results in Cancer Research. Berlin, Heidelberg: Springer; 2003. doi: 10.1007/978-3-642-59349-9_13
40. Di Trapani M, Manaresi N, Medoro G. DEPArray™ system: An automatic image-based sorter for isolation of pure circulating tumor cells. Cytometry A. 2018; 93(12): 1260-1266. doi: 10.1002/cyto.a.23687
41. Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007; 450(7173): 1235-1239. doi: 10.1038/nature06385
42. Placke T, Örgel M, Schaller M, et al. Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res. 2012; 72(2): 440-448. doi: 10.1158/0008-5472.CAN-11-1872
43. Andree KC, van Dalum G, Terstappen LW. Challenges in circulating tumor cell detection by the CellSearch system. Mol Oncol. 2016; 10(3): 395-407. doi: 10.1016/j.molonc.2015.12.002
44. Riethdorf S, Fritsche H, Müller V, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: A validation study of the CellSearch system. Clin Cancer Res. 2007; 13(3): 920-928. doi: 10.1158/1078-0432.CCR-06-1695
45. Zhang L, Riethdorf S, Wu G, et al. Meta-analysis of the prognostic value of circulating tumor cells in breast cancer. Clin Cancer Res. 2012; 18: 5701-5710. doi: 10.1158/1078-0432.CCR-12-1587
46. Bidard F-C, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: A pooled analysis of individual patient data. Lancet Oncol. 2014; 15(4): 406-414. doi: 10.1016/S1470-2045(14)70069-5
47. Miller MC, Doyle GV, Terstappen LW. Significance of circulating tumor cells detected by the CellSearch system in patients with metastatic breast colorectal and prostate cancer. J Oncol. 2010; 2010: 617421-617421. doi: 10.1155/2010/617421
48. Balic M, Dandachi N, Hofmann G, et al. Comparison of two methods for enumerating circulating tumor cells in carcinoma patients. Cytometry B Clin Cytom. 2005; 68B(1): 25-30. doi: 10.1002/cyto.b.20065
49. Allen JE, El-Deiry WS. Circulating tumor cells and colorectal cancer. Curr Colorectal Cancer Rep. 2010; 6(4): 212-220. doi: 10.1007/s11888-010-0069-7
50. Fehm T, Müller V, Aktas B, et al. HER2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast Cancer Res Treat. 2010; 124(2): 403-412. doi: 10.1007/s10549-010-1163-x
51. Maly V, Maly O, Kolostova K, Bobek V. Circulating tumor cells in diagnosis and treatment of lung cancer. In Vivo. 2019; 33(4): 1027-1037. doi: 10.21873/invivo.11571
52. Matthew EM, Zhou L, Yang Z, et al. A multiplexed markerbased algorithm for diagnosis of carcinoma of unknown primary using circulating tumor cells. Oncotarget. 2016; 7(4): 3662-3676. doi: 10.18632/oncotarget.6657
53. Wong SC, Chan CM, Ma BB, et al. Clinical significance of cytokeratin 20-positive circulating tumor cells detected by a refined immunomagnetic enrichment assay in colorectal cancer patients. Clin Cancer Res. 2009; 15(3): 1005-1012. doi: 10.1158/1078-0432. CCR-08-1515
54. Wong SCC, Ng SSM, Cheung MT, et al. Clinical significance of CDX2-positive circulating tumour cells in colorectal cancer patients. Br J Cancer. 2011; 104(6): 1000-1006. doi: 10.1038/ bjc.2011.32
55. Alix-Panabières C, Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016; 6(5): 479-491. doi: 10.1158/2159-8290.CD-15-1483
56. Marrinucci D, Bethel K, Bruce RH, et al. Case study of the morphologic variation of circulating tumor cells. Hum Pathol. 2007; 38(3): 514-519. doi: 10.1016/j.humpath.2006.08.027
57. Marrinucci D, Bethel K, Luttgen M, Bruce RH, Nieva J, Kuhn P. Circulating tumor cells from well-differentiated lung adenocarcinoma retain cytomorphologic features of primary tumor type. Arch Pathol Lab Med. 2009; 133(9): 1468-1471. doi: 10.1043/1543- 2165-133.9.1468
58. Demay RM. The Art & Science of Cytopathology. 2nd ed. Chicago, USA: ASCP Press; 2011.
59. Hardingham JE, Hewett PJ, Sage RE, et al. Molecular detection of blood-borne epithelial cells in colorectal cancer patients and in patients with benign bowel disease. Int J Cancer. 2000; 89(1): 8-13.
60. Pantel K, Denève E, Nocca D, et al. Circulating epithelial cells in patients with benign colon diseases. Clin Chem. 2012; 58(5): 936- 940. doi: 10.1373/clinchem.2011.175570
61. Motherby H, Nadjari B, Friegel P, Kohaus J, Ramp U, Böcking A. Diagnostic accuracy of effusion cytology. Diagn Cytopathol. 1999; 20(6): 350-357. doi: 10.1002/(sici)1097- 0339(199906)20:6<_x0033_50:_x003a_aid-dc5>3.0.co;2-7
62. Cakir E, Demirag F, Aydin M, Unsal E. Cytopathologic differential diagnosis of malignant mesothelioma, adenocarcinoma and reactive mesothelial cells: A logistic regression analysis. Diagnos Cytopathol. 2009; 37(1): 4-10. doi: 10.1002/dc.20938
63. Light RW. Pleural effusion. N Eng J Med. 2002; 346(25): 1971- 1977. doi: 10.1056/NEJMcp010731
64. Light RW, Erozan YS, Ball WC. Cells in pleural fluid: Their value in differential diagnosis. JAMA Intern Med. 1973; 132: 854-860.
65. Prakash UBS, Reiman HM. Comparison of needle biopsy with cytologic analysis for the evaluation of pleural effusion: Analysis of 414 cases. Mayo Clin Proc. 1985; 60(3): 158-164. doi: 10.1016/ s0025-6196(12)60212-2
66. Pai R, Shenoy K, Minal J, Suresh P, Chakraborti S, Lobo F. Use of the term atypical cells in the reporting of ascitic fluid cytology: A caveat. Cytojournal. 2019; 16(1): 13. doi: 10.4103/cytojournal.cytojournal_37_18
67. Roh MH. Triage of cytologic direct smears for ancillary studies: A case-based illustration and review. Arch Pathol Lab Med. 2013; 137(9): 1185-1190. doi: 10.5858/arpa.2013-0235-CR
68. Kim L, Tsao MS. Tumour tissue sampling for lung cancer management in the era of personalised therapy: What is good enough for molecular testing? Eur Respir J. 2014; 44(4): 1011-1022. doi: 10.1183/09031936.00197013
69. Malapelle U, Bellevicine C, Zeppa P, Palombini L, Troncone G. Cytology-based gene mutation tests to predict response to antiepidermal growth factor receptor therapy: A review. Diagn Cytopathol. 2011; 39(9): 703-710. doi: 10.1002/dc.21512
70. Nicholson AG, Gonzalez D, Shah P, et al. Refining the diagnosis and EGFR status of non-small cell lung carcinoma in biopsy and cytologic material, using a panel of mucin staining, TTF-1, cytokeratin 5/6, and P63, and EGFR mutation analysis. J Thorac Oncol. 2010; 5(4): 436-441. doi: 10.1097/JTO.0b013e3181c6ed9b
71. Bochtler T, Krämer A. Does cancer of unknown primary (CUP) truly exist as a distinct cancer entity? Front Oncol. 2019; 9: 402. doi: 10.3389/fonc.2019.00402
72. Travis WD, Rekhtman N, Riley GJ, et al. Pathologic diagnosis of advanced lung cancer based on small biopsies and cytology: A paradigm shift. J Thorac Oncol. 2010; 5(4): 411-414. doi: 10.1097/JTO.0b013e3181d57f6e
73. da Cunha Santos G, Saieg MA, Geddie W, Leighl N. EGFR gene status in cytological samples of nonsmall cell lung carcinoma. Cancer Cytopathol. 2011; 119(2): 80-91. doi: 10.1002/cncy.20150
74. Sun P-L, Jin Y, Kim H, Lee C-T, Jheon S, Chung J-H. High concordance of EGFR mutation status between histologic and corresponding cytologic specimens of lung adenocarcinomas. Cancer Cytopathol. 2013; 121(6): 311-319. doi: 10.1002/cncy.21260
75. Knoepp SM, Roh MH. Ancillary techniques on direct-smear aspirate slides. Cancer Cytopathol. 2013; 121(3): 120-128. doi: 10.1002/cncy.21214
76. Roy-Chowdhuri S. From cytopathology in focus: Updated NSCLC guideline moves molecular cytopathology forward. CAP Today. Website. https://www.captodayonline.com/cytopathology-infocus-updated-nsclc-guideline-moves-molecular-cytopathology-forward/. Accessed October 14, 2019.
77. Chinen LTD, de Carvalho FM, Rocha BMM, et al. Cytokeratinbased CTC counting unrelated to clinical follow up. J Thorac Dis. 2013; 5(5): 593-599. doi: 10.3978/j.issn.2072-1439.2013.09.18
78. Ferreira MM, Ramani VC, Jeffrey SS. Circulating tumor cell technologies. Mol Oncol. 2016; 10(3): 374-394. doi: 10.1016/j.molonc.2016.01.007
79. Zheng S, Lin HK, Lu B, et al. 3D microfilter device for viable circulating tumor cell (CTC) enrichment from blood. Biomed Microdevices. 2011; 13(1): 203-213. doi: 10.1007/s10544-010-9485-3
80. Lin E, Cao T, Nagrath S, King MR. Circulating tumor cells: Diagnostic and therapeutic applications. Ann Rev Biomed Eng. 2018; 20(1): 329-352. doi: 10.1146/annurev-bioeng-062117-120947
81. Peeters DJE, De Laere B, Van den Eynden GG, et al. Semiautomated isolation and molecular characterisation of single or highly purified tumour cells from CellSearch enriched blood samples using dielectrophoretic cell sorting. Br J Cancer. 2013; 108(6): 1358- 1367. doi: 10.1038/bjc.2013.92
82. Baker MK, Mikhitarian K, Osta W, et al. Molecular detection of breast cancer cells in the peripheral blood of advanced-stage breast cancer patients using multimarker real-time reverse transcriptionpolymerase chain reaction and a novel porous barrier density gradient centrifugation technology. Clin Cancer Res. 2003; 9(13): 4865- 4871.
83. Gabriel MT, Calleja LR, Chalopin A, Ory B, Heymann D. Circulating tumor cells: A review of non–EpCAM-Based approaches for cell enrichment and isolation. Clin Chem. 2016; 62(4) :571-581. doi: 10.1373/clinchem.2015.249706
84. Bankó P, Lee SY, Nagygyörgy V, et al. Technologies for circulating tumor cell separation from whole blood. J Hematol Oncol. 2019; 12(1): 48. doi: 10.1186/s13045-019-0735-4
85. Harouaka RA, Nisic M, Zheng SY. Circulating tumor cell enrichment based on physical properties. J Lab Autom. 2013; 18(6): 455-468. doi: 10.1177/2211068213494391
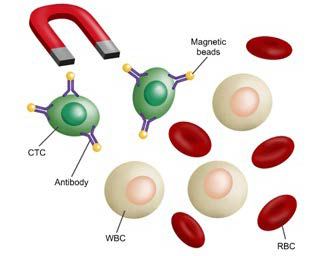
86. Lustberg M, Jatana KR, Zborowski M, Chalmers JJ. Emerging technologies for CTC detection based on depletion of normal cells. Recent Results Cancer Res. 2012; 195: 97-110. doi: 10.1007/978- 3-642-28160-0_9
87. Yin J, Wang Y, Yin H, et al. Circulating tumor cells enriched by the depletion of leukocytes with bi-antibodies in non-small cell lung cancer: Potential clinical application. PLoS One. 2015; 10(8): e0137076-e0137076. doi: 10.1371/journal.pone.0137076