INTRODUCTION
Campylobacter is one of the most frequently reported food-borne pathogens and causes an estimated 1.3 million infections in the United States annually.1While the majority of Campylobacter cases result in acute gastroenteritis, infection has also been associated with more severe diseases, including Guillain-Barré syndrome, Reactive arthritis (ReA), Irritable Bowel Syndrome (IBS), and Inflammatory Bowel Disease (IBD).2Epidemiological evidence indicates that the most common source for Campylobacter infections in humans is due to consumption of poultry products.3 This is typically caused by the consumption of improperly cooked chicken or cross-contamination from handling raw chicken.1,4 Campylobacter colonization in poultry is common; as many as 90% of US broiler flocks are contaminated with this food-borne pathogen.5 Therefore, a reduction or elimination of Campylobacter in poultry is a research priority to reduce the risk of infection in humans. Many pre-harvest strategies have been evaluated for reducing Campylobacter in poultry, such as bio-security, probiotics, competitive exclusion, bacteriocins, bacteriophages, vaccines, and natural compounds, often with limited success.6 Recently, the natural product chitosan has shown potential to reduce colonization of another food-borne pathogen, Salmonella Typhimurium, in pre-harvest poultry7 and may have application against Campylobacter. Chitosan has also shown efficacy against other Gram-negative species, including Escherichia coli and Pseudomonas fluorescens.8,9 Chitosan, a natural by-product derived from the deacetylation of chitin, is obtained from crab and shrimp shell waste.10,11 Chitosan is a potential natural food preservative with broad antimicrobial benefits.8,12,13 Although the exact mode of action of chitosan is not completely understood, researchers have previously determined that chitosan is capable of interacting with the outer cell membrane of bacterial pathogens, altering its permeability, disrupting cellular physiology and causing cell death.9,14 To our knowledge, the ability of chitosan to reduce Campylobacter colonization in poultry has not been evaluated. The purpose of this study was to determine the efficacy of in-feed supplementation of chitosan on Campylobacter colonization in broiler chicks. Young chickens were used in this study because previous results from our laboratory demonstrated that young birds can be used as a reliable model to study Campylobacter colonization in market age birds.15,16
MATERIALS AND METHODS
Chitosan Materials
Chitosan of molecular weight 50-190 kDa and 190-310 kDa was obtained from Sigma-Aldrich (St. Louis, MO, USA), and 400-600 kDa chitosan was purchased from Spectrum Chemicals (New Brunswick, NJ, USA).
In vitro Susceptibility of C. jejuni to Chitosan
Antimicrobial activity of each molecular weight chitosan, low (50-190 kDa), medium (190-310 kDa) and high (400-600 kDa), in a 0.5% (wt./vol.) solution was determined by inoculating each solution with a four-strain mixture of wildtype C. jejuni. Preparation of the Campylobacter inoculum was done as described previously by Farnell and others.17 In brief, working stock cultures of the four wild-type strains of C. jejuni were obtained by individually inoculating each strain into fresh Campylobacter Enrichment Broth (CEB, Acumedia, Neogen Corporation, Lansing, MI, USA) from frozen glycerol stock and successively sub-culturing twice at 42 ºC for 48 h under microaerophilic conditions. Strain mixtures were then combined centrifuged at 3000 * g for 10 minutes and the cell pellet re-suspended in 10 mL Butterfield’s Phosphate Diluent (BPD). A 1% stock solution (wt./vol.) of each molecular weight of chitosan was prepared in 50 mM acetic acid as described by Ganan and others.18 For the experiment, the stock concentration of each of the chitosan solutions and the acetic acid control was diluted 1:1 with an inoculum containing 108 CFU/mL of C. jejuni, resulting in a final concentration of 0.5% for each chitosan. Sample time points included 0, 2, 4 and 8 h post inoculation. At each time point, an aliquot from the treatments and control was taken and 1:10 serial dilutions were direct plated on Campy Line Agar.19 The plates were incubated for 48 h at 42 ºC in a microaerophilic atmosphere. Direct enumeration of Campylobacter colonies was converted to CFU/mL for each treatment. Each susceptibility assay was repeated in duplicate.
In vivo Susceptibility of C. jejuni to Chitosan
Day of hatch Cobb broiler chicks (Siloam Springs, AR, USA) from a local commercial hatchery were utilized for the animal experiments. In each of three replicate trials, 100 chicks per trial were randomly divided into 10 treatments, which consisted of three concentrations (0.25%, 0.5%, or 1% wt./wt.) of each molecular weight chitosan, which was added to the feed and a positive control (0% chitosan). Birds were placed in floor pens and provided feed and water ad libitum; treated feed was provided throughout the entire trial.
The Campylobacter challenge was prepared as mentioned above. Birds were challenged by oral gavage with 0.25 mL of a four-strain mixture of wild-type C. jejuni on day 6, at a concentration of 107 -108 CFU/mL. On day 15, birds were euthanized and the ceca were excised for Campylobacter enumeration. Cecal contents were serially diluted 10-fold with BPD and plated on CLA for direct enumeration. Plates were incubated at 42 ºC under microaerophilic conditions for 48 h and enumerated for Campylobacter colonies as previously described by our laboratory.20 All the experiments conducted in this study were approved by the Institutional Animal Care and Use Committee of the University of Arkansas.
Chitosan Solution Preparation and Determination of Sub-Inhibitory Concentration (SIC)
The chitosan solution was prepared as mentioned previously.18 The SIC of chitosan was determined using previously published protocol.21 Briefly, 24 well polystyrene plates (Costar, Corning, NY, USA) containing CEB (2 mL/well) supplemented with two-fold dilutions of chitosan (0, 0.2, 0.1, 0.05, 0.025, 0.0125 and 0.00625%), were inoculated with ~5.0 log CFU of C. jejuni wild strain, followed by incubation at 42 ºC for 24 h. Bacterial growth was determined by culturing on CLA agar plates. The highest concentration of chitosan that did not inhibit C. jejuni growth during mid-log (8 h), and stationary phase (24 h) were selected as the SIC for the compound.
RNA Isolation, cDNA Synthesis and Real-time Quantitative PCR
The effect of SIC of chitosan on the expression of Campylobacter genes critical for colonization in chicken was investigated using real-time quantitative PCR (RT-qPCR), as described previously.21The wild type C. jejuni strain was randomly selected from the four strains used in the in vivo trials for gene expression analysis. The strain was cultured with or without SIC of chitosan at 42 ºC in CEB to mid-log phase (8 h) and total RNA was extracted using the RN easy Mini kit (Qiagen, Valencia, CA, USA), followed by complementary DNA synthesis (iScript cDNA synthesis kit, Bio-Rad). The cDNA synthesized was used as the template for RT-qPCR. The amplification product was detected using SYBR Green reagent (iQ SYBR Green Supermix, Bio-Rad). The primers for each gene (Table 1) were designed from published GeneBank C. jejuni sequences using Primer 3 software National Center for Biotechnology Information (NCBI) and synthesized from IDT DNA. The relative expression of candidate genes was determined using the comparative critical threshold (∆∆Ct) method on a Quant Studio 3 real-time PCR system (Applied Biosytems, Thermo Fisher Scientific, Carlsbad, CA, USA). Data were normalized to the endogenous control (16S rRNA), and the level of expression of target genes between treated and untreated samples were analyzed to study effect of chitosan on expression of each gene. Duplicate samples were used and the assay was repeated three times.
Table 1: Primers used for real time quantitative PCR (RT-qPCR) analysis.
|
Gene with Accession no.
|
Primer
|
Sequence (5’- 3’)
|
Gene description
|
|
16S-rRNA
(NC_002163.1)
|
Forward
Reverse
|
5’-TGAGGGAGAGGCAGATGGAA-3’ 5’-TCGCCTTCGCAATGGGTATT-3’
|
Ribosomal RNA (housekeeping gene)
|
|
cadF
(NC_002163.1)
|
Forward
Reverse
|
5’-CGCGGGTGTAAAATTCCGTC-3’ 5’-TCCTTTTTGCCACCAAAACCA-3’
|
Outer membrane fibronectin-binding protein
|
|
jlpA
(NC_002163.1)
|
Forward
Reverse
|
5’-AGCACACAGGGAATCGACAG-3’ 5’-TAACGCTTCTGTGGCGTCTT-3’
|
Surface exposed lipoprotein
|
|
ciaB
(NC_002163.1)
|
Forward
Reverse
|
5’-TCTCAGCTCAAGTCGTTCCA-3’ 5’-GCCCGCCTTAGAACTTACAA-3’
|
Invasion antigen protein
|
|
fliA
(NC_002163.1)
|
Forward
Reverse
|
5’-AGCTTTCACGCCGTTACGAT-3’ 5’-TCTTGCAAAACCCCAGAAGT-3’
|
Flagella biosynthesis RNA polymerase sigma factor
|
|
motA
(NC_002163.1)
|
Forward
Reverse
|
5’-AGCGGGTATTTCAGGTGCTT-3’ 5’-CCCCAAGGAGCAAAAAGTGC-3’
|
Flagellar motor protein
|
|
motB
(NC_002163.1)
|
Forward
Reverse
|
5’-AATGCCCAGAATGTCCAGCA-3’ 5’-AGTCTGCATAAGGCACAGCC-3’
|
Flagellar motor protein
|
Statistical Analysis
Cecal Campylobacter counts were logarithmically transformed before analysis to achieve homogeneity of variance.22 Analysis of the data was done using the PROC GLM procedure of SAS.23 Treatment means were partitioned by LSMEANS analysis and probability of p<0.05 was required for statistical significance. Data comparisons for the gene expression study were performed using multiple t-test with GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla California, USA, www.graphpad.com).
RESULTS
Chitosan in vitro
Campylobacter counts were reduced by approximately 1 log at 2 and 4 h when co-incubated with 0.5% for all three molecular weights of chitosan as compared with controls (Table 2). At 8 h, all three chitosan preparations produced a 4.5 to 5 log reduction in counts when compared with controls.
Table 2: The effect of different molecular weight chitosans on growth of Campylobacter jejuni in vitro1,2,3 Campylobacter counts, in vitro.
10.5% concentration of: low molecular weight chitosan is 50-190 kDa; medium molecular weight chitosan is 190-310 kDa; or high molecular weight chitosan is 400-600 kDa, in 50 mM acetic acid.
2Campylobacter inoculum was added to each chitosan treatment and sampled at 0, 2, 4, and 8 h; samples were plated and enumerated after 48 h incubation.
3Values represent average campylobacteriosis counts of two separate replicate trials.
|
Treatment
|
Time in hours
|
|
0
|
2 |
4 |
8
|
|
Positive controls
|
6.35*107
|
8.15*107 |
5.45*107 |
3.5*107
|
|
Low Molecular Weight
|
3.42*107
|
6.8*106 |
1.24*106 |
3.0*102
|
|
Medium Molecular Weight
|
8.55*107
|
2.55*106 |
1.82*106 |
5.5*102
|
|
High Molecular Weight
|
7.45*107
|
2.59*106 |
2.00*106 |
6.5*102
|
Chitosan in vivo
In trial 1, Campylobacter counts were reduced in six of the chitosan treatments: 0.25% and 0.5% Low Molecular Weight (LMW), 0.25% and 0.5% Medium Molecular Weight (MMW), 0.25% and 1% High Molecular Weight (HMW), in comparison to the positive control (Table 3). Trial 2 showed a significant reduction of Campylobacter by four of the chitosan treatments: 0.5% LMW, 1% LMW, 0.25% MMW, and 0.5% MMW (Table 3). Results from Trial 3 showed a significant reduction of Campylobacter by one of the chitosan treatments: 0.5% MMW (Table 3).
Table 3: The effect of different concentrations and molecular weight chitosans on cecal Campylobacter jejuni counts (means±SEM) in 15-day old broiler chicks during three separate trials1,2,3,4 Campylobacter counts, in vivo.
1Low molecular weight chitosan is 50-190 kDa; medium molecular weight chitosan is 190-310 kDa; high molecular weight chitosan is 400-600 kDa.
2ND: non-detectible.
3Day-of-hatch birds were fed chick starter treatments of 0.25%, 0.5% or 1% of either low molecular weight, medium molecular weight or high molecular weight chitosan, respectively, for the entire 15-day study; bird were inoculated with Campylobacter jejuni mixture on Day 6 and cecal contents were collected on Day 15 for campylobacteriosis enumeration.
4 Means within columns with no common superscript differ significantly (p<0.05).
|
Chitosan dose
|
Trial 1 |
Trial 2 |
Trial 3
|
|
Positive controls Control
|
0%
|
8.77±.17a |
7.05±.69a |
8.36±.24 a
|
|
Low Molecular Weight
|
0.25%
|
7.06±.58cde |
7.1±.29ab |
8.59±.20 a
|
|
0.5%
|
7.68±.27 bcd |
3.96±1.02c |
7.88±.38ab
|
|
1.0%
|
7.96±.15abc |
NDd |
7.76±.40 ab
|
|
Medium Molecular Weight
|
0.25%
|
6.76±.34de |
4.83±1.08bc |
8.47±.21 a
|
|
0.5%
|
7.4±.38bcd |
3.25±.94c |
7.28±.70b |
| 1.0% |
8.03±.14 abc |
7.45±.34 a |
8.57±.17 a
|
|
High Molecular Weight
|
0.25%
|
7.45±.19 bcd |
7.49±.31 a |
8.16±.29 ab
|
|
0.5%
|
8.43±.18 ab |
7.8±.35 a |
8.34±.26 a |
| 1.0% |
6.3±.74e |
7.31±.30 a |
8.51±.19 a
|
SIC and Gene Expression Analysis
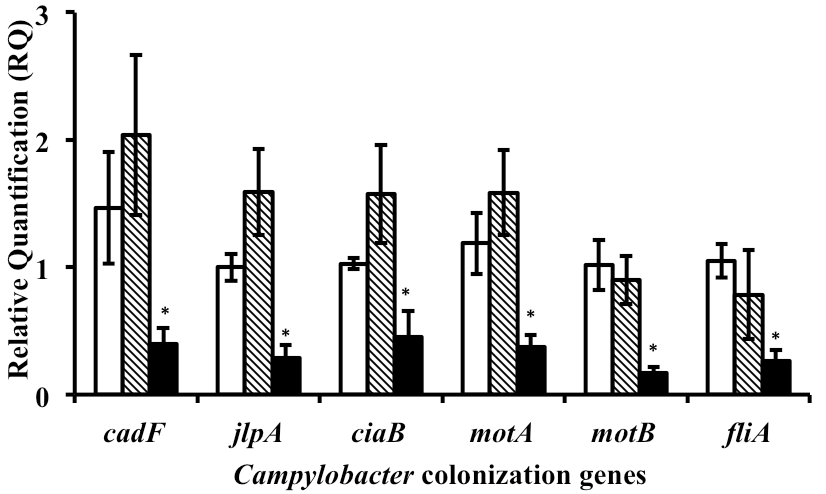
Since MMW chitosan was found to consistently reduce Campylobacter counts in vivo; we selected MMW chitosan for the gene expression analysis. One of the C. jejuni wild strain used in the in vivo study was randomly selected for the mechanistic study. Based on growth curve results (data not shown), the SIC of MMW chitosan that did not inhibit C. jejuni strain growth as compared to control was 0.0125%. This concentration of MMW chitosan was used for subsequent gene expression analysis. RT-qPCR results (Figure 1) revealed that MMW chitosan significantly reduced the transcription of genes coding for Campylobacter motility; namely, fliA, motA, motB and adherence (cadF, jlpA, ciaB) as compared to control (P<0.05). The expression of chicken colonization genes was not significantly affected by acetic acid (P>>0.05)
DISCUSSION
Preliminary in vitro results utilizing a 0.5% dose demonstrate that the three molecular weight chitosan treatments reduce Campylobacter counts in comparison to the untreated controls (Table 2). To evaluate the ability of chitosan to reduce enteric Campylobacter colonization in chickens, the 0.5% concentration of all three molecular weight chitosans, plus a lower (0.25%) and higher dose (1%) were also evaluated. In the first trial, cecal Campylobacter counts were reduced in 6 out of 8 of the treatments (Table 3). When conducted in a second trial, 4 of the 8 treatments were effective; whereas in the third replicate trial, the 0.5% MMW reduced enteric Campylobacter counts when compared with controls (Table 3). Although there is variability between replicate trials, the 0.5% MMW chitosan dose consistently reduced Campylobacter in all three trials.
To determine the potential mechanism of action of chitosan, we investigated the effect of SICs of MMW chitosan on the expression of critical chicken colonization genes of Campylobacter. SICs of antimicrobials, including antibiotics are known to alter pathophysiology of microbes by modulating gene transcription.21,24,25,26 In C. jejuni, the flagellar biosynthesis gene, fliA regulates a large number of genes involved in motility, protein synthesis and colonization.27A mutation in fliA has been shown to reduce motility and colonization potential in chicken cecum.28 Similarly, motA, motB are critical for flagella motor function and facilitate motility and colonization.27 CadF is another important virulence gene that encodes a 37 kDA outer membrane protein, that along with CiaB and JlpA, promotes adherence to intestinal cells and colonization in the avian intestinal tract.27,29 We observed that SIC of MMW chitosan significantly decreased the expression of motility genes as well as adherence genes as compared to control (Figure 1), indicating that the anti-colonization effect of chitosan could be potentially mediated through reduced transcription of critical genes.
Figure 1: Effect of 0.0125% MMW chitosan on the expression of chicken colonization genes (means ±SEM) in Campylobacter jejuni.1
The importance of replicating results demonstrating a significant reduction in enteric Campylobacter counts in preharvest poultry cannot be underestimated. Previous research conducted by our laboratory15,20,30 and others31,32,33 have highlighted the variability between trials when evaluating pre-harvest treatments against enteric Campylobacter. Because of this inherent variability associated with Campylobacter colonization studies, results from a single pre-harvest study may not fully evaluate the consistency or effectiveness of a Campylobacter intervention strategy.34,35,36,37
Feed application of chitosan is a viable application for reducing Campylobacter colonization in chickens; however, water application is also a possible option. Unfortunately, chitosan is insoluble in water within the normal pH range.38,39 This problem can be resolved by mildly acidifying the water, as accomplished in our in vitro studies. It is possible this will enhance the efficacy of chitosan as proposed by Qin and co-workers.39 Acidifying water lines is already being performed in some poultry operations, which can reduce another foodborne pathogen, Salmonella;22 thereby, this aids in the reduction of Campylobacter as well in the water lines and during feed withdrawal prior to processing, without altering the gut epithelium.40,41 Thus, acidifying water in poultry houses could have a number of positive effects on bird health and reduce the potential zoonotic transfer of pathogens to humans. This possibility is currently under investigation.
The use of pre-harvest intervention strategies to reduce Campylobacter colonization (e.g., chitosan) can be part of a multifaceted approach to reduce the incidence of this foodborne pathogen. It has been proposed that a 2-log reduction in Campylobacter on the chicken carcass could reduce the risk of human campylobacteriosis by up to 30-fold.42 Perceivably “small” reductions of Campylobacter in chickens could result in large reductions of campylobacteriosis incidences in humans. Olson and colleagues compiled data relevant to the consistent rise of campylobacteriosis incidences from the 1980’s through 2006 as occurred in many countries, including Denmark, England, Wales, Norway, Sweden, New Zealand, and Australia, many of which are currently monitored by the European Centre for Disease Prevention and Control (ECDC).43 In the 2000’s, New Zealand focused on poultry as the primary source of Campylobacter, and by applying required regulatory implementations, along with the assistance of voluntary interventions, New Zealand saw a 54% decline in campylobacteriosis incidences in 2008, compared to the period 2002-2006.44 This decline was associated with a reduction in Campylobacter counts in chicken meat.45 New Zealand’s well-documented reduction of campylobacteriosis cases sets precedence for global reduction of Campylobacter by focusing intervention strategies on the poultry industry.
In conclusion, enteric Campylobacter counts were consistently reduced by in-feed supplementation of 0.5% MMW chitosan in three replicate trials. The use of this chitosan in pre-harvest poultry may be incorporated into a multifaceted strategy to reduce Campylobacter counts in chickens. Also, chitosan can be used to further reduce or inhibit Campylobacters surviving on the chicken carcass or meat. Further studies are warranted to explore the potential use of chitosan for reducing Campylobacter contamination in pre- and post-harvest poultry and the potential mechanism of action through whole transcriptome analysis.
ACKNOWLEDGEMENTS
Funded in part by the USDA-NIFA-OREI 2011-01955.
CONFLICTS OF INTEREST
Mention of a trade name, proprietary product, or specific equipment does not constitute a guarantee or warranty by the USDA and does not imply its approval to the exclusion of other products that are suitable.
The authors declare that they have no conflicts of interest.






