INTRODUCTION
Ovarian stromal luteinization has been observed in a number of female pelvic pathologies ranging from the benign to malignant, and predominantly in mucinous neoplasms.1 Its occurrence is associated with an increase in estrogen secretion and has often been reported in endometrial carcinoma.2,3,5 We report an unusual case of high-grade papillary serous carcinoma of the ovary with stromal luteinization in a 67-year old postmenopausal woman. The morphologic appearance of this tumor was somewhat similar to Sertoli-Leydig cell tumor of the ovary. This report emphasizes that stromal luteinization can be encountered in any epithelial ovarian tumors, including serous carcinoma, and may mimic ovarian sex cord stromal tumors. Interestingly, this patient also had an independent primary uterine papillary serous carcinoma of the endometrium.
CASE PRESENTATION
A 67-year-old gravida 0 presented with a history of lower abdominal pain for one month and vaginal spotting for 3 weeks. The pain was dull in nature, initially intermittent and became constant. She also noticed abnormal bloating, early satiety, 10-pounds weight gain, and altered bowel movement in two weeks prior to presentation. She attained menarche at 9 years and menopause at 52 years. Her past medical history included hypertension, gastro-esophageal reflux, and basal cell carcinoma. Bimanual examination revealed definite palpable bilateral adnexal masses, which were minimally tender with mass effect, abutting the rectum.
A pelvic ultrasound revealed a large 11 cm cystic and solid mass in the left adnexa and a 5.5 cm adnexal mass on the right side. Ascites was noted. The 6.3×3.3×2.8 cm uterus revealed 12 mm thick endometrial stripe on ultrasound examination. CA-125 was elevated at 1202. CT scan confirmed the findings of the ultrasound. Omental thickening noted on CT scan.
Prior to laparotomy, an endometrial biopsy was done and it demonstrated a high-grade papillary serous carcinoma and Endometrial Intraepithelial Carcinoma (EIC). Because of an endometrial serous carcinoma with bilateral adnexal masses and possible omental disease, she underwent total abdominal hysterectomy, bilateral salpingo-oophorectomy and omentectomy.
Histologic examination of the uterus, right ovary, left ovary, omentum and plaques from sigmoid colon surface and left pelvic side-wall all showed high-grade papillary serous carcinoma with psammoma bodies and necrosis. The psammoma bodies and necrosis were also seen in the tumors of ovaries, omentum, and all serosal lesions. The findings raised a question whether the patient has an advanced IVB endometrial carcinoma or two independent primary tumors of the ovary and endometrium? The following unique features noted in the histologic sections which guided the final diagnoses: The serous carcinoma of the endometrium was superficially invasive and associated with endometrial intraepithelial carcinoma (EIC), a feature that justifies a primary endometrial serous carcinoma designation. In addition, no lymph vascular space invasion was noted in the uterus which would have supported a diagnosis of an advanced endometrial carcinoma. Similarly, the ovarian serous carcinoma had features that were consistent with tumor arising from the epithelial inclusion cysts in the ovary. No serous carcinoma, invasive or in-situ, was seen in the fallopian tube fimbria or in the mucosa. The distribution of the serous carcinoma in this case was typical of a primary ovarian carcinoma, which involved bilateral ovaries, serosa of bilateral fallopian tubes, uterine serosa, bilateral pelvic walls, sigmoid colon serosa, and the omentum. In the absence of an in-situ and invasive carcinoma, either in the fimbria or mucosa of the fallopian tube, it appeared prudent to assign the ovary as second primary site in this patient. The omental tumor deposits measured 0.5 to 1.0 cm in maximum dimensions and no gross omental caking.
Although the two primary serous carcinomas in the same individual are not uncommon, the most unusual feature, and the basis of this case report, was noted in the right ovary. The strikingly prominent stromal luteinization around the invasive serous tumor was seen in bilateral ovaries (Figure 1). Immunohistochemical staining with p53 and inhibin-alpha showed the tumor cells were strongly and diffusely reactive to p53 whereas, the luteinized stroma cells were reactive to inhibin (Figure 1). Figure 2 shows Invasive and in-situ serous carcinoma of the ovary in the left and endometrium in the right hand column respectively.
Figure 1: High-grade papillary serous carcinoma with marked stromal luteinization (H&E x 10) left had column. p53 immunostaining (x20), right column, upper. Inhibin immunostaining (x10), right column, lower.
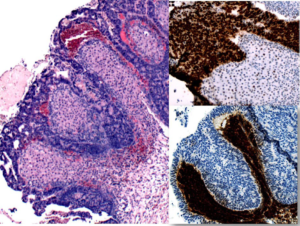
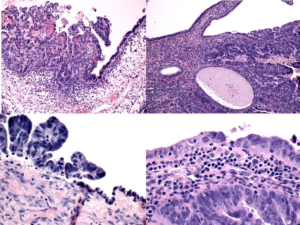
Figure 2: High-grade papillary serous carcinoma, ovary (H&E 10x, left upper; H&E 20x left lower column). High grade serous carcinoma, endometrium (H&E, 10x, right upper; H&E 20x, right lower). Both photomicrographs showing evidence that these tumors are arising from these sites.
DISCUSSION
Stromal Luteinization has been described to be associated with ovarian mucinous and endometrioid neoplasms.2-5 Rome, et al. observed that stromal luteinization in postmenopausal women with mucinous ovarian tumors often has higher than normal estrogen secretion.2
It has been postulated that a mutation of GT198 protein (a tumor suppressor gene and steroid hormone receptor) in ovarian stromal cell results in stromal activation with resultant luteinization due to the presence of luteinized theca cells at different developmental stage.6
These mutant stromal cells are often found in endometrioid, mucinous, serous, clear cell and granulosa cell tumors, and as well as, endometrial and metastatic gastrointestinal adenocarcinoma.3,7 A few isolated cases of stromal luteinization has been reported in primary B cell lymphoma of the ovary,8 ovarian carcinoid,9 ovarian teratomas,10 ovarian hemangiomas11 and Brenner tumors.6 However, the significance of these luteinized stromal cells is largely unknown.
High-grade serous carcinoma constitute 60%-80% of ovarian epithelial carcinoma while ovarian cancer in general accounts for only 3% of all cancers in women.12 Despite its high prevalence, the serous carcinoma of the ovary is usually not associated with stromal luteinization. The morphologic appearance of this tumor may be confused with a sertoli-leydig cell tumor of the ovary. At a lower magnification, the admixture of epitheliallike element and luteinized stromal cells created a morphologic appearance that is often seen in Seroli-Leydig cells. However, the strong p53 positivity in the epithelial component of the ovarian tumor differentiated this tumor from a sertoli-leydig cell tumor. This is an important differential diagnosis as the prognosis and treatment of these two entities is vastly different. Immunostaining can confirm the presence of luteinization of ovarian stromal cells. The luteinized stromal cells are positive for alpha Inhibin, Calretinin and Melan A.9
In conclusion, stromal luteinization may be seen in high-grade serous epithelial ovarian tumors as demonstrated in this case and such occurrence should not be overlooked or confused with other tumors by the astute surgical pathologist.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
ETHICAL APPROVAL
The patient has provided written consent for the use of the images and case presentation for educational and scientific purposes provided unique identification is not revealed.
AUTHORSHIP ROLE
1. Mopupeola O. Samalia, MBBS: (Fellow in training and wrote the case report)
Fellow, Stuart Lauchlan Fellowship in Gynecologic and Breast Pathology, Women & Infants Hospital/Alpert Medical School of Brown University, Providence, RI 02905, USA.
2. C. James Sung, MD: (Helped writing the case report)
Program Director, Stuart Lauchlan Fellowship in Gynecologic and Breast Pathology, Women & Infants Hospital/Alpert Medical School of Brown University, Providence, RI 02905, USA.
3. W. Dwayne Lawrence, MD, MSc (Path) (Reviewed the case and helped in diagnosis)
Chief of Pathology, Stuart Lauchlan Fellowship in Gynecologic and Breast Pathology, Women & Infants Hospital/Alpert Medical School of Brown University, Providence, RI 02905, USA.
4. M. Ruhul Quddus, MD, M. Phil (Path) (Attending on the case)
Program Co-Director, Stuart Lauchlan Fellowship in Gynecologic and Breast Pathology, Women & Infants Hospital/Alpert Medical School of Brown University, Providence, RI 02905, USA.







