1. Pendsey SP. Understanding diabetic foot. Int J Diabetes Dev Ctries. 2010; 30(2): 75-79. doi: 10.4103/0973-3930.62596
2. Kerr M, Barron E, Chadwick P, et al. The cost of diabetic foot ulcers and amputations to the National Health Service in England. Diabet Med. 2019; 36(8): 995-1002. doi: 10.1111/dme.13973
3. Mottolini N. Diabetes and lower limb complications: A thematic review of clinical negligence claims. 2022. Website. https://resolution.nhs.uk/resources/diabetes-and-lower-limb-complications-a-thematic-review-of-clinical-negligence-claims/.Accessed September 26, 2022.
4. Shu J, Santulli G. Update on peripheral artery disease: Epidemiology and evidence-based facts. Atherosclerosis. 2018; 275: 379-381. doi: 10.1016/j.atherosclerosis.2018.05.033
5. Guest J, Fuller GW, Vowden P. Diabetic foot ulcer management in clinical practice in the UK: Costs and outcomes. Int Wound J. 2018; 15: 43-52. doi: 10.1111/iwj.12816
6. McInnes W, Jeffcoate W, Vileikyte L, et al. Foot care education in patients with diabetes at low risk of complications: A consensus statement. Diabet Med. 2011; 28: 162-167. doi: 10.1111/j.1464-5491.2010.03206.x
7. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017; 376(24): 2367-2375. doi: 10.1056/NEJMra1615439
8. Moulik PK, Mtonga R, Gill GV. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care. 2003; 26(2): 491-494. doi: 10.2337/diacare.26.2.491
9. Kosiborod M, Gomes MB, Nicolucci A, et al. Vascular complications in patients with type 2 diabetes: Prevalence and associated factors in 38 countries (the DISCOVER study program). Cardiovasc Diabetol. 2018; 150(15): 150. doi: 10.1186/s12933-018-0787-8
10. Hamilton MT, Hamilton DH, Zderic TW. Sedentary behaviour as a mediator of type 2 diabetes. Med Sport Sci. 2014; 60: 11-26. doi: 10.1159/000357332
11. Colberg SR, Sigal RJ, Fernhall Bo, et al. Exercise and type 2 diabetes – The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care. 2010; 33: e147-e167. doi: 10.2337/dc10-9990
12. Schaper NC, van Netten JJ, Apelqvist J, Bus SA, Hinchliffe RJ, Lipsky BA. IWGDF Guidelines on the prevention and management of diabetic foot disease. The International Working Group on the Diabetic Foot. 2019. Website. https://iwgdfguidelines.org/wp-content/uploads/2019/05/IWGDF-Guidelines-2019. pdf. Accessed September 26, 2022.
13. Morbach S, Furchert H, Groblinghoff U, et al. Long-term prognosis of diabetic foot patients and their limbs: Amputation and death over the course of a decade. Diabetes Care. 2012; 35:2021-2027. doi: 10.2337/dc12-0200
14. Suryani M, Samekto W, Nugroho H, Susanto H, Dwiantoro L. Effect of foot-ankle flexibility and resistance exercise in the secondary prevention of plantar foot diabetic ulcer. J Diabetes Complications. 2021; 35(9): 107968. doi: 10.1016/j.jdiacomp.2021.107968
15. LeMaster JW, Mueller MJ, Reiber GE, Mehr DR, Madsen RW, Conn VS. Effect of weight-bearing activity on foot ulcer incidence in people with diabetic peripheral neuropathy: Feet first randomized controlled trial. Phys Ther. 2008; 88(11): 1385-1398. doi: 10.2522/ptj.20080019
16. Nwankwo MJ, Okoye GC, Victor EA, Obinna EA. Effect of twelve weeks supervised aerobic exercise on ulcer healing and changes in selected biochemical profiles of diabetic foot ulcer subjects. International Journal of Diabetes Research. 2014; 3(3): 41-48. doi: 10.5923/j.diabetes.20140303.03
17. Nwankwo MJ, Okoye GC, Victor EA, Obinna EA. The effect of twelve weeks supervised aerobic exercise intervention on lower extremities oxygenation and wound healing among diabetic ulcer subjects. International Journal of Diabetes Research. 2017; 6(3): 47-53. doi: 10.5923/j.diabetes.20170603.01
18. Lindberg K, Moller BS, Kirketerp-Moller K, Kristensen MT. An exercise program for people with severe peripheral neuropathy and diabetic foot ulcers – a case series on feasibility and safety. Disabil Rehabil. 2020; 42(2): 183-189. doi: 10.1080/09638288.2018.1494212
19. Holdcroft A. Gender bias in research: How does it affect evidence based medicine? J R Soc Med. 2007; 100(1): 2-3. doi: 10.1258/jrsm.100.1.2
20. Andrade C. The inconvenient truth about convenience and purposive samples. Indian J Psychol Med. 2021; 43(1): 86-88. doi:
10.1177/0253717620977000
21. Pal K, Horsfall L, Sharma M, Nazareth I, Petersen I. Time trends in the incidence of clinically diagnosed type 2 diabetes and pre-diabetes in the UK 2009-2018: A retrospective cohort study. BMJ Open Diabetes Res Care. 2018; 9(1): e001989. doi: 10.1136/bmjdrc-2020-001989
22. Song SH. Complication characteristics between young-onset type 2 versus type 1 diabetes in a UK population. BMJ Open Diabetes Res Care. 2015; 3(1): e000044. doi: 10.1136/bmjdrc-2014-000044
23. Brownrigg JRW, Apelqvist J, Bakker K, Schaper NC, Hinchliffe RJ. Evidence-based management of PAD & the diabetic foot. Eur J Vasc Endovasc Surg. 2013; 45(6): 673-681. doi: 10.1016/j.ejvs.2013.02.014
24. Butterworth PA, Urquhart DM, Landorf KB, Wluka AE, Cicuttini FM. Foot posture, range of motion and plantar characteristics in obese and non-obese individuals. Gait Posture. 2014; 41:465-469. doi: 10.1016/j.gaitpost.2014.11.010
25. Vijayaraghavan K. Treatment of dyslipidemia in patients with type 2 diabetes. Lipids Health Dis. 2010; 9: 144. doi: 10.1186/1476-511X-9-144
26. Al-Goblan AS, Al-Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes. 2014; 7: 587-591. doi: 10.2147/DMSO.S67400
27. Baker C. Obesity Statistics. 2022. Websit. https://researchbriefings.files.parliament.uk/documents/SN03336/SN03336.pdf. Accessed September 30, 2022.
28. Szpalski C, Wetterau M, Cohen O, et al. Obesity impairs wound healing and neovasculogenesis. Plastic and Reconstructive Surgery. 2011; 128(Supplement 4S): 50. doi: 10.1097/01.prs.0000406269.87317.b6
29. Ghadieh AS, Saab B. Evidence for exercise training in the management of hypertension in adults. Can Fam Physician. 2015; 61: 233-239.
30. Liu M, Zhang W, Yan Z, Xiangzhen Y. Smoking increases the risk of diabetic foot amputation: A meta-analysis. Exp Ther Med. 2018; 15: 1680-1685. doi: 10.3892/etm.2017.5538
31. Hariton E, Locascio JJ. Randomised controlled trials-the gold standard for effectiveness research. BJOG. 2018; 125(13): 1716-1716. doi: 10.1111/1471-0528.15199
32. Schroeder EC, Franke WD, Sharp RL, Lee DC. Comparative effectiveness of aerobic, resistance, and combined training on cardiovascular disease risk factors: A randomised controlled trial. PLoS One. 2019; 14(1): e0210292. doi: 10.1371/journal.pone.0210292
33. Balducci S, Zanuso S, Nicolucci A, et al. Effect of an intensive exercise intervention strategy on modifiable cardiovascular risk factors in subjects with type 2 diabetes mellitus a randomized controlled trial: The Italian diabetes and exercise study. Arch Intern Med. 2010; 170(20): 1794-1803. doi: 10.1001/archinternmed.2010.380
34. Hardcastle SJ, Taylor AH, Bailey MP, Harley RA, Hagger MS. Effectiveness of a motivational interviewing intervention on weight loss, physical activity and cardiovascular disease risk factors: A randomised controlled trial with a 12-month post-intervention follow up. Int J Behav Nutr Phys Act. 2013; 10: 40. doi: 10.1186/1479-5868-10-40
35. Irvine C, Taylor NF. Progressive resistance exercise improves glycaemic control in people with type 2 diabetes mellitus: A systematic review. Aust J Physiother. 2009; 55(4): 237-246. doi: 10.1016/s0004-9514(09)70003-0
36. Lakicevic N, Gentile A, Mehrabi S, et al. Make fitness fun: Could novelty be the key determinant for physical activity adherence? Front Psychol. 2020; 11: 577522. doi: 10.3389/fpsyg.2020.577522
37. Packer CF, Ali SA, Manna B. Diabetic Ulcer. Treasure Island, Florida, USA: StatPearls; 2021.
38. Mohamed O, Cerny K, Jones W, Burnfield JM. The effect of terrain on foot pressures during walking. Foot Ankle Int. 2005; 26(10): 859-869. doi: 10.1177/107110070502601012
39. Masuadi E, Mohamud M, Almutairi M, Alsunaidi A, Alswayed AK, Aldhafeeri OF. Trends in the usage of statistical software and their associated Study Designs in Health Sciences Research: A bibliometric analysis. Cureus. 2021; 13(1): e12639. doi: 10.7759/cureus.12639
40. Zeng C, Luo S, Xu S, Li Y. The application of DOMS mechanism and prevention in physical education and training. J Healthc Eng. 2022; 2022: 9654919. doi: 10.1155/2022/9654919
41. DeFronzo R, Fleming A, Chen K, Bicsak TA. Metformin associated lactic acidosis: Current perspectives on causes and risk. Metabolism. 2016; 65: 20-29. doi: 10.1016/j.metabol.2015.10.014
42. Bair MJ, Brizendine EJ, Ackermann RT, Shen C, Kroenke K, Marrero DG. Prevalence of pain and association with quality of life, depression and glycaemic control in patients with diabetes. Diabet Med. 2010; 27: 579-584. doi: 10.1111/j.1464-5491.2010.02971.x
43. Armstrong DA, Lipsky BA. Diabetic foot infections: Stepwise medical and surgical management. Int Wound J. 2004; 1: 123-132. doi: 10.1111/j.1742-4801.2004.00035.x
44. Abbema RV, De Greef M, Craje C, Krijnen W, Hobbelen H, Van Der Schans C. What type, or combination of exercise can improve preferred gait speed in older adults? A meta-analysis. BMC Geriatrics. 2015; 15: 72. doi: 10.1186/s12877-015-0061-9
45. World Health Organization (WHO). Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus. 2011. Website. https://apps.who.int/iris/bitstream/handle/10665/70523/WHO_NMH_CHP_CPM_11.1_eng.pdf. Accessed September 30, 2022.
46. Papathanasiou G, Georgakopoulos D, Papageorgiou E, et al. Effects of smoking on heart rate at rest and during exercise, and on heart rate recovery, in young adults. Hellenic J Cardiol. 2013; 54: 168-177.
47. Mann S, Beedie C, Jimenez A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: Review, synthesis and recommendations. Sports Med. 2014; 44: 211-221. doi: 10.1007/s40279-013-0110-5
48. Williams DT, Harding KG, Price PE. The influence of exercise perfusion in diabetes. Diabet Med. 2007; 24: 1105-1111. doi: 10.1111/j.1464-5491.2007.02218.x
49. Flahr D. The effect on nonweight-bearing exercise and protocol adherence on diabetic foot ulcer healing: A pilot study. Ostomy Wound Manage. 2010; 56(10): 40-50.
50. Najafi B, Grewal GS, Bharara M, Menzies R, Talal TK, Armstrong DG. Can’t stand the pressure: The association between unprotected standing, walking, and wound healing in people with diabetes. J Diabetes Sci Technol. 2017; 11(4): 657-667. doi: 10.1177/1932296816662959
51. Mueller MJ, Maluf KS. Tissue adaptation to physical stress: A proposed “Physical Stress Theory” to guide physical therapist practice, education, and research. Phys Ther. 2002; 82(4): 383-403. doi: 10.1093/ptj/82.4.383
52. Lin JH, Jeon SY, Romano PS, Humphries MD. Rates and timing of subsequent amputation after initial minor amputation. J Vasc Surg. 2020; 72(1): 268-275. doi: 10.1016/j.jvs.2019.10.063
53. Kizilkurt OK, Kizilkurt T, Gulec MY, Giynas FE, Polat G, Kilicoglu OI, Gulec H. Quality of life after lower extremity amputation due to diabetic foot ulcer: The role of prosthesis-related factors, body image, self-esteem, and coping styles. Dusunen Adam The Journal of Psychiatry and Neurological Sciences. 2020; 33: 109-119. doi: 10.14744/DAJPNS.2020.00070
54. Qui S, Cai X, Schumann U, Velders M, Sun Z, Steinacker JM. Impact of walking on glycemic control and other cardiovascular risk factors in type 2 diabetes: A meta-analysis. PLoS One. 2014; 9(10): e109767. doi: 10.1371/journal.pone.0109767
55. Holt RIG. Diabetes and depression. Curr Diab Rep. 2014; 14(6): 491. doi: 10.1007/s11892-014-0491-3
56. Chen ML, Lin B-S, Su CW, et al. The application of wireless near infrared spectroscopy on detecting peripheral circulation in patients with diabetes foot ulcer when doing Buerger’s exercise. Lasers Surg Med. 2017; 49: 652-657. doi: 10.1002/lsm.22667
57. Boden WE, Franklin B, Berra K, et al. Exercise as a therapeutic intervention in patients with stable ischemic heart disease: An underfilled prescription. Am J Med. 2014; 127: 905-911. doi:
10.1016/j.amjmed.2014.05.007
58. Ignaszewski M, Lau B, Wong S, Isserow S. The science of exercise prescription: Martti Karvonen and his contributions. BC Medical Journal. 2017; 59(1): 38-41.
59. Hap K, Biernat K, Konieczny G. Patients with diabetes complicated by peripheral artery disease: The current state of knowledge on physiotherapy interventions. J Diabetes Res. 2021; 2021: 5122494. doi: 10.1155/2021/5122494
60. Bus SA, Armstrong DG, Gooday CF, Viswanathan W, Lazzarini PA. IWGDF Guidelines on offloading foot ulcers in persons with diabetes. International Working Group on the Diabetic Foot (IWGDF). 2020; 36 (S1). doi: 10.1002/dmrr.3274
61. Treat-Jacobson D, McDermott MM, Bronas UG, et al. Optimal exercise programs for patients with peripheral artery disease a scientific statement from the American Heart Association. Circulation. 2019; 139: e10-e33. doi: 10.1161/CIR.0000000000000623
62. Zaharieva DP, Riddell MC. Prevention of exercise-associated dysglycemia: A case study-based approach. Diabetes Spectr. 2015; 28(1): 55-62. doi: 10.2337/diaspect.28.1.55
63. Cordell CB, Borson S, Boustani M, et al. Alzheimer’s Association recommendations for operationalizing the detection of cognitive impairment during the Medicare Annual Wellness Visit in a primary care setting. Alzheimers Dement. 2013; 9: 141-150. doi: 10.1016/j.jalz.2012.09.011
64. Kumar A, Cannon CP. Acute coronary syndromes: Diagnosis and management, part I. Mayo Clin Proc. 2009; 84(100): 917-938. doi: 10.1016/S0025-6196(11)60509-0
APPENDICES
| Table 1. Carbohydrate Intake Strategies Based on Pre-Exercise Blood Glucose Level62 |
| Pre-Exercise Blood Glucose Concentration Author |
Requirements |
| < 90 mg/dL |
Ingest 15-30 g of fast-acting carbohydrates before the onset of exercise, depending on the size of the individual. Follow with extra carbs throughout exercise. |
| 90-149 mg/dL |
Start consuming extra carbs at the onset of exercise (~0.5-1.0 g/kg body mass/hour of exercise), depending on the energy expenditure and the amount of circulating insulin at the time of exercise |
| 150-249 mg/dL |
Initiate exercise and delay consumption of extra carbs until blood glucose levels drop to< 150 mg/dL |
| 250-349 mg/dL |
Test for ketones: do not perform any exercise if moderate amounts of ketones are present58; contact your health care team. Initiate mild- to moderate-intensity exercise. Intense exercise should be delayed until glucose levels drop to< 250 mg/dL because intense exercise may exaggerate the huperglycemia. |
| ≥350 mg/dL |
Test for ketones: do not perform any exercise if moderate to large amounts of ketones are present58; contact your health care team. If ketones are negative (or trace), consider conservative insulin correction (e. g., 50% correction) before exercise, depending on current “on board” (active) insulin status. Initiate mild to moderate exercise and avoid intense exercise (aerobic of anaerobic) until glucose levels drop |
| Blood glucose concentrations should always be checked before exercise, and if glucose is dramatically elevated (≥350 mg/dL), the urine or blood should also be tested for ketones. The target range for blood glucose before exercise is 90-250 mg/dL. Carbohydrate intake should depend on the glucose concentration at the start of exercise. Regardless of their initial blood glucose concentration, patients should continue to monitor blood glucose regularly during exercise (every 30-45-minutes) using an accurate glucose meter and to adjust insulin and carbohydrate intake accordingly. In general, adjusting insulin doses before exercise will reduce the need for increased carbohydrate intake. Adapted from Holt55, Ignaszewski et al58 |
| Table 2. Study Selection |
| Study Selection |
Requirements |
| Population |
Human participants with type 1 or 2 diabetes with peripheral neuropathy and peripheral arterial disease and an active or recently healed plantar foot ulcer at time of study. |
| Intervention |
Aerobic exercise combined with resistance-based exercises or solely aerobic exercise programme lasting longer than 10 weeks in duration. |
| Outcomes |
Cardiorespiratory, glycaemic control, cholesterol and lipids, wound-healing, ankle brachial index, circulatory toe pressures, patient satisfaction scores. |
Table 3. Participant Compatibles Nr*=Non-recorded
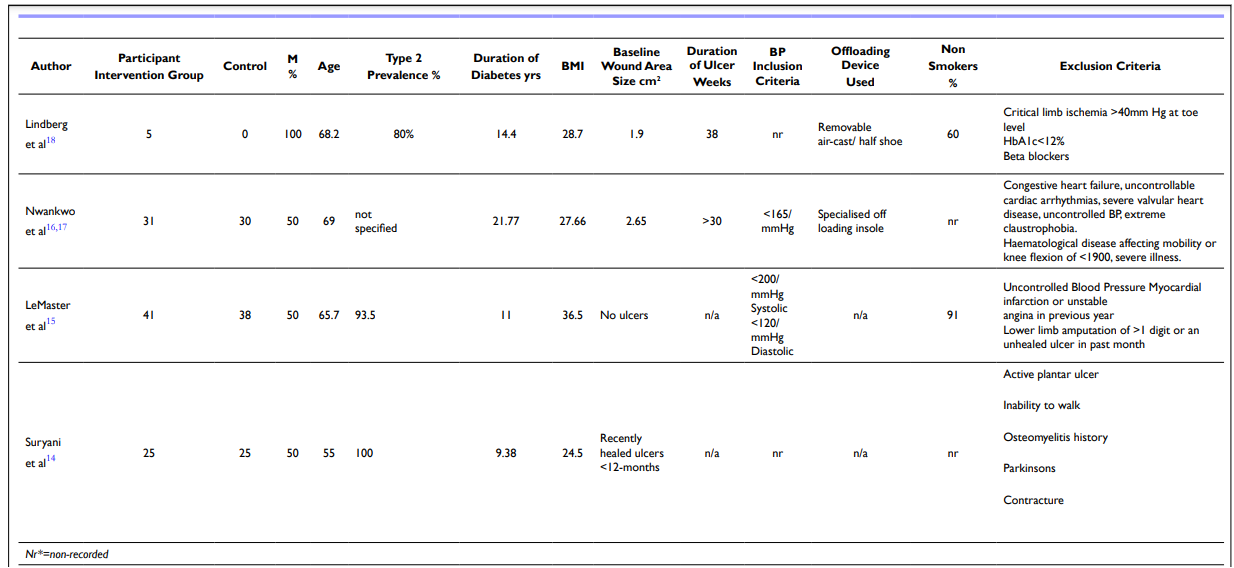
Table 4. Methods
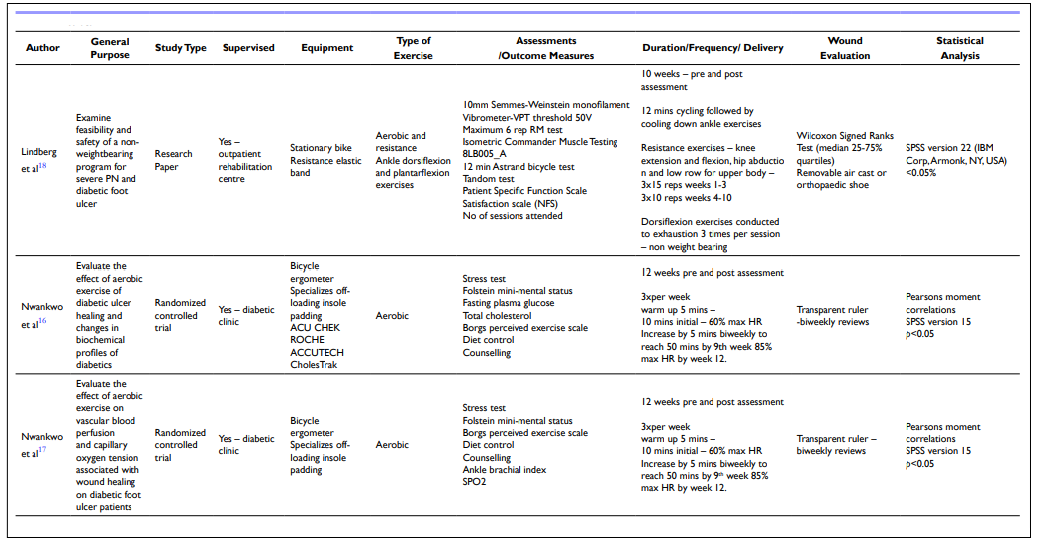
| Table 5. Results |
| Authors |
Adverse Reactions |
Adherence |
Results |
| Lindberg et al18 |
1 participant suffered from frozen shoulder pain and needed breaks.
3 out of 5 participants suffered from DOMS which compromised programme.
2 participants had extubate of ulcer.
2 participants had habitual back pain. |
All patients completed programme – 92% average attendance. |
At 10 weeks
• 3 of 5 ulcers had healed and all had decreased in size.
• Ability to complete daily living activities improved.
• NRS score median 10.
• Patient satisfaction score had improved.
• All muscle groups had improved strength, mostly in hip abduction.
• Tandem and HBA1c inconclusive. |
| Nwankwo et al16 |
|
No participants absconded or died |
At 12 weeks
• Decrease in fasting plasma glucose in experimental group.
• No difference in total cholesterol blood levels.
• Wound size percentage reduction larger in experimental group |
| Nwankwo et al17 |
|
No participants absconded or died |
At 12 weeks
• Increase in SPO2 in both groups but more in experimental group.
• Increase in ABI in experimental group.
• Wound size reduction with no correlation to BMI after 4-weeks in experimental group.5
• Wound size reduction at 12 weeks in experimental group with a connection to BMI.
• In control group no connection between BMI, wound size and SPO2 . |
| LeMaster et al15 |
57 lesions detected in total.
4 ulcers in intervention group >1cmsq
1 ulcer in control group >1cmsq
Average duration of ulcer in intervention–74 days
Average duration of ulcer in control – 51.5 days.
1 participant suffered proximal phalangeal great toe fracture due to osteoporosis |
1 person died from unrelated cause
Average sessions during first 6 months in intervention group – 3×per week. Average sessions during first 6 months in control – 1.5-days per week.
At 12-months average sessions per well for both groups was 1.5 sessions. |
At 6 months
• Steps increased during 30-minute sessions by 14% in intervention group.
• Overall steps increased by 2.5% in intervention group.
• Overall steps decreased by 6% in control group.
At 12 months
• Steps decreased between 6-12 months for intervention groups during 30-minute exercise and overall daily steps.
• Overall steps decreased for control group. |
| Suryani et al14 |
|
6 participants did not complete programme.
No participants died.
According to logbook, very good
compliance in the intervention group |
At 12 weeks
• Ulcers in 2 patients (4%) in intervention group.
• Ulcers in 17 patients (68%) in control group.
• DNE decreased in intervention group. • Walking speed increased in intervention group.
• HBA1C level unchanged in both groups.
• ABI no significant difference in both groups but did increase gradually in intervention group.
At 24 weeks
• Ulcers in 4 patients (16%) in intervention group.
• Ulcers in 17 patients (72%) in control group.
• DNE decreased in intervention group.
• Walking speed increased in intervention group.
• ABI no significant difference in both groups but did increase gradually in intervention group. |
|
Table 6. Participant Criteria
|
|
Inclusion Criteria
|
Exclusion Criteria
|
Aged 40-70-years
Male or Female
Non-smoking status
Diagnosed peripheral neuropathy
Mild to Moderate Ischemia (ABI levels 0.6 hmm and above combined with TBPA toe
pressure of 40-59 hmm12
Controlled blood pressure< 180/110 mmHg29
Active plantar foot ulcer classification of grade 2 or below on the Wagner scale
Infection level of< 1 and below12
Cognitive function will be predicted to be sufficient by their primary physician based
on MiniCog assessment tool63
Patients wear a removable offloading walker on the affected foot
|
ABI, < 0.6, toe pressure, 40 hmm
Presence of gangrene, osteomyelitis, serious illness. Wagner score >2
Infection level >112
Unstable blood pressure or BP >180/11 mmHg
Unstable cardiovascular angina64 Cognitive function predicted to be insufficient based on Mini Cog assessment tool by primary their primary physician. |
| Table 7. Participant Criteria |
| Karvonen Example Method |
| (220)-(your age)=MaxHR (MaxHR)-(mean resting heart rate)=HRR (HRR)x(60% to 80%)=training range % (Training range %)+(mean resting heart rate)=(target training zone) |
| Table 8. Outcome Measures |
| Baseline |
6-Weeks |
12-Weeks |
Pre-activity level questionnaire
Semmes monofilament test
SPO2 levels
TBPI toe pressures
ABI levels
Blood Pressure
Height
Weight
BMI
Wound size–transparent ruler Fasting blood glucose
Blood cholesterol
Removable walker check |
Patient Satisfaction Survey
Semmes monofilament test
SPO2 levels
TBPI toe pressures
ABI levels
Blood Pressure
Height
Weight
BMI
Wound size – transparent ruler Fasting blood glucose
Blood cholesterol
Removable walker check |
Patient Satisfaction Survey Semmes monofilament test SPO2 levels
TBPI toe pressures
ABI levels
Blood Pressure
Height
Weight
BMI
Wound size – transparent ruler
Fasting blood glucose
Blood cholesterol Removable walker check |
| Table 9. Programme Timetable to be Provided by the Physician |
| |
Monday |
Tuesday |
Wednesday |
Thursday |
Friday |
Saturday Sunday |
| Week 1 |
2 minute warm up – 20-30% HRR
5 mins rowing – 30-40% HRR
2×30 reps –
Leg extension
Hamstring curl
Adductor
Abductor
* Stretching programme |
|
2 minute warm up – 20-30% HRR 5 mins cycling – 30-40% HRR
2×30 reps –
Chest press Shoulder
press
Abdominal crunch
*Stretching programme |
|
2 min warm up -20-30% HRR
8 mins rowing – 30-40% HRR
*Stretching programme |
|
| Week 2 |
2 minute warm up – 20-30% HRR
8 mins cycling – 30-40% HRR
2×30 reps –
Leg extension
Hamstring curl
Adductor
Abductor
*Stretching programme |
|
2 minute warm up –20-30% HRR
10 mins rowing – 30-40% HRR
2×30 reps –
Chest press
Shoulder press
Abdominal crunch
*Stretching programme |
|
2 minute warm up – 20-30% HRR
10 mins cycling -30-40% HRR
Diabetic nurse review and
motivational interviewing
*Stretching programme |
|
| Week 3 |
3 minute warm up – 30-40% HRR
10 mins rowing – 40-50% HRR
2×30 reps –
Leg extension
Hamstring curl
Adductor
Abductor
*Stretching programme |
|
3 minute warm up –30-40% HRR
10 mins cycling – 40-50% HRR
2×30 reps –
Chest press
Shoulder press
Abdominal crunch
*Stretching programme |
|
3 minute warm up – 30-40% HRR
12 mins rowing 40-50% HRR
*Stretching programme |
|
| Week 4 |
3 minute warm up – 30-40% HRR
12 mins cycle 40-50% HRR
2×30 reps –
Leg extension
Hamstring curl
Adductor
Abductor
*Stretching programme |
|
3 minute warm up – 30-40% HRR
15 mins rowing – 40-50% HRR
2×30 reps
Chest press
Shoulder press
Abdominal crunch
*Stretching programme |
|
3 minute warm up – 30-40% HRR
15 mins cycling – 40-50% HRR
Diabetic nurse review
*Stretching programme |
|
| Week 5 |
4 minute warm up
30-40% HRR
18 mins rowing – 40-50% HRR
2×15 reps
Leg extension
Hamstring curl
Adductor
Abductor
Chest press
Shoulder press
Abdominal crunch
*Stretching programme |
|
4 minute warm up
30-40% HRR
18 mins cycling – 40-50% HRR
2×15 reps
Leg extension
Hamstring curl
Adductor
Abductor
Chest press
Shoulder press
Abdominal crunch
*Stretching programme |
|
4 minute warm up 30-40% HRR 18 mins rowing 40-50% HRR
*Stretching programme |
|
| Week 6 |
4 minute warm up
30-40% HRR
20 mins cycling – 40-50% HRR
2×15 reps
Leg extension
Hamstring curl
Adductor
Abductor
Chest press
Shoulder press
Abdominal crunch
*Stretching programme |
|
4 minute warm up
30-40% HRR
20 mins rowing – 40-50% HRR
2×15 reps
Leg extension
Hamstring curl
Adductor
Abductor
Chest press
Shoulder press
Abdominal crunch
*Stretching programme |
|
4 minute warm up 30-40% HRR 20 mins cycling 40-50% HRR
*Stretching programme
Diabetic nurse review and motivational interviewing
Assessments |
|
| Week 7 |
5 minute warm up
30-40% HRR
20 mins rowing – 40-60% HRR
2×15 reps
Leg extension
Hamstring curl
Adductor
Abductor
Chest press
Shoulder press
Abdominal crunch
*Stretching programme |
|
5 minute warm up
30-40% HRR
20 mins cycling – 40-60% HRR
2×15 reps
Leg extension
Hamstring curl
Adductor
Abductor
Chest press
Shoulder press
Abdominal crunch
*Stretching programme |
|
5 minute warm up 30-40% HRR 20 mins rowing 40-60% HRR
*Stretching programme |
|
| Week 8 |
5 minute warm up
30-40% HRR
22 mins cycling – 40-60% HRR
2×15 reps
Leg extension
Hamstring curl
Adductor
Abductor
Chest press
Shoulder press
Abdominal crunch
*Stretching programme |
|
5 minute warm up
30-40% HRR
22 mins rowing – 40-60% HRR
2×15 reps
Leg extension
Hamstring curl
Adductor
Abductor
Chest press
Shoulder press
Abdominal crunch
*Stretching programme |
|
5 minute warm up 30-40% HRR 22 mins cycling 40-60% HRR Diabetic nurse review
*Stretching programme |
|
| Week 9 |
5 minute warm up
30-40% HRR
25 mins rowing – 40-60% HRR
2×12 reps
Leg extension
Hamstring curl
Adductor
Abductor
Chest press
Shoulder press
Abdominal crunch
*Stretching programme |
|
5 minute warm up
30-40% HRR
25 mins cycling – 40-60% HRR
2×12 reps
Leg extension
Hamstring curl
Adductor
Abductor
Chest press
Shoulder press
Abdominal crunch
*Stretching programme |
|
5 minute warm up 30-40% HRR 25 mins rowing 40-60% HRR
*Stretching programme |
|
| Week 10 |
5 minute warm up
30-40% HRR
28 mins cycling – 40-60% HRR
2×12 reps
Leg extension
Hamstring curl
Adductor
Abductor
Chest press
Shoulder press
Abdominal crunch
*Stretching programme |
|
5 minute warm up
30-40% HRR
28 mins rowing – 40-60% HRR
2×12 reps
Leg extension
Hamstring curl
Adductor
Abductor
Chest press
Shoulder press
Abdominal crunch
*Stretching programme |
|
5 minute warm up 30-40% HRR 28 mins cycling 40-60% HRR Diabetic nurse review and motivational interviewing
*Stretching programme |
|
| Week 11 |
5 minute warm up
30-40% HRR
30 mins rowing – 40-60% HRR
2×12 reps
Leg extension
Hamstring curl
Adductor
Abductor
Chest press
Shoulder press
Abdominal crunch
*Stretching programme |
|
5 minute warm up
30-40% HRR
30 mins cycling – 40-60% HRR
2×12 reps
Leg extension
Hamstring curl
Adductor
Abductor
Chest press
Shoulder press
Abdominal crunch
*Stretching programme |
|
5 minute warm up 30-40% HRR 30 mins rowing 40-60% HRR
*Stretching programme |
|
| Week 12 |
5 mins warm up
30-50% HRR
30 mins cycling – 50-60% HRR
2×12 reps
Leg extension
Hamstring curl
Adductor
Abductor
Chest press
Shoulder press
Abdominal crunch
*Stretching programme |
|
5 minute warm up
30-50% HRR
30 mins rowing – 50-60% HRR
2×12 reps
Leg extension
Hamstring curl
Adductor
Abductor
Chest press
Shoulder press
Abdominal crunch
*Stretching programme |
|
5 mins warm up 30-50% HRR 30 mins cycling 50-60% HRR
*Stretching programme Diabetic nurse review Assessments |
|
HRR=Heart Rate Reserve
* Stretching programme – sat on exercise mat, hold each stretch for 30 seconds, repeat on both sidesx2.
Seated hamstring stretch, seated inner thigh stretch, kneeling quadricep stretch, kneeling hip flexor stretch, seated triceps stretch, seated deltoid stretch |
| Table 10. Budget and Justification |
| Personnel |
Hours/ Amount |
Cost |
| Level 4 Advanced Exercise Trainer |
139.5 hours at £25 |
£3487.50 |
| Band 5 Orthotist plus allowance for prescribed diabetic shoes |
19 hours at £11.23 |
£213.37 |
| Band 7 Diabetic nurse/ wound specialist |
50 hours at £16.83 |
£841.50 |
| Researcher |
|
|
| Band 2 Porter to transport pa-tients around hospital |
150 hours at £8.61 |
£1291.50 |
Rehabilitation Gym Hire
To be included in clinic hire costs:
Recumbent bike
Rowing machine
Leg press machine
Hamstring curl machine
Abdominal crunch machine
Chest Press machine
Shoulder press machine
Exercise mats
Stadiometer
Digital weighing scales |
144 hours at £50 |
£5400.00 |
| Diabetic Clinic room |
7-days estimated costs at £200 |
£1400.00 |
| Equipment |
Make/Model |
Cost |
| Handheld – 8mHz doppler in-strument |
Hi Dop Vascular Doppler with 8 MHz Probe |
|
| SPO2 pulsometer |
MediSupplies Finger Pulse Oximeter MD300C2 |
|
| 10 g Monofilament instrument |
Bailey Retractable Monofila-ment 10 g |
|
| Glucometer/ Ketometer |
Glucomen Areo Glucometer and Ketometer |
|
| Glucose testing strips – 2×50 |
Glucomen – 2×50 at £14.29 |
|
| Ketone testing strips – 2×10 |
Glucomen – 2×10 at £14.29 |
|
| Cholesterol meter |
GlucoRx X6 Multi Parameter Meter |
|
| Cholesterol strips |
GlucoRx X6 Total Cholester-ol Testing Strips 10×10 at £29.95 |
|
| Polar heart rate monitor chest strap |
Polar H10 H HR Sensor BLE BLK M-XXLx5 at £66 |
|
| Transparent wound ruler |
McKesson Wound measuring guide 5×7 inch clear plastic disposable–100 packx2 at £10.99 |
|
| Blood Pressure machine |
Reister RBP–100 Blood Pressure Monitor Mobile De-vice |
|
| Lenovo Yoga Laptop |
7 Pro X 14.5 inch slim |
|
| SPSS software (version 28) |
6 months subscription |
|
| Sphygmomanometry |
Atys SysToe |
|
| Pedometer |
Besportable 3D Digital Pe-dometer–30x£13.59 |
|
| Stopwatch |
Kalenji Onstart 110 Stop-watch |
|
| Supplies |
Amount |
Cost |
Water from water machine
Glucogel 3xtubes
Carbohydrate snacks
Apple juice
Paper towels
Disinfectant
Logbook to document adverse events and attendance rates
Pens
Print out of exercise programme |
|
Estimate allowance of £200.00 |
| Travel |
Hours/ Amount |
Cost |
| Patient travel allowance – ambulance or taxi collection and drop off up to 5 miles distance from hospital |
Allowance of £15 per person per day |
£9000.00 |
Total cost: £27079.54
Total cost adding 40% margin
to staff costs: £29413.09 |







