INTRODUCTION
Intraosseous ablation of the basivertebral nerve (BVNA) is an emerging minimally invasive treatment to relieve chronic mechanical axial low back pain associated with Modic type 1, or Modic type 2, vertebral end-plate changes.1,2,3 The BVNA procedure is supported by a multi-center, prospective, randomized, double-blind sham-controlled trial with results now published out to 5 years4,5,6 and by a multi-centre randomized trial.7
The results reported include statistically significant decreases in pain, measured with the numerical rating scale (NRS) or the visual analog scale (VAS), and improvements in function, measured with the oswestry disability index (ODI).
Historically, obtaining sustainable and effective treatment outcomes for patients with chronic mechanical axial low back pain associated with Modic type 1 or type 2 vertebral end-plate changes has been challenging.
The inclusion criteria used in the published trials included chronic low back pain of more than six months duration, an inadequate response to six months or more of conservative care, and Modic type 1 or type 2 vertebral end-plate changes at one or more segments from L3 to S1. Additional inclusion criteria included the presence of dysfunction, as measured by an ODI greater than 30, and intrusive pain, as measured by an NRS greater than 4.4,5,6,7
Wright et al8 devised and described a novel bi-pedicular technique after appropriate benchwork to demonstrate the potential effectiveness, safety and patient outcomes following bipolar radiofrequency ablation (RFA) of the basivertebral nerve (BVN) by positioning transpedicular radiofrequency electrodes to bracket the intravertebral target tissue.9 The reported case continues to experience relief of symptoms more than seven years later (personal communication, Dr Wright).
This case report describes the application of a bi-pedicular bipolar radiofrequency technique for BVNA, including the early outcomes and MRI findings.
CASE REPORT
A 40-year-old woman presented with eight years of central low back pain, with mechanical features, occasionally radiating to the buttocks and posterior thighs and with no radiation below the knees. Previous management included pharmacotherapy (oxycodone 50 mg daily), physiotherapy and exercise programme (including Pilates) and breast reduction (which helped cervicothoracic spinal pain symptoms). Background history of treated anxiety and depression and previous lobular breast carcinoma-in-situ (incidental finding at surgical excision for breast reduction) with no residual disease at 4-year follow-up.
The pain intensity was worse in the morning, constantly present and aggravated by activity, particularly by coughing and sneezing. There were no red flag symptoms such as unintended weight loss, fevers or recumbency pain. Pain severity was rated as averaging 8/10 over the previous week using the NRS.
Examination disclosed height 165 cm and weight 72 kg. Gait was mildly antalgic. The posture was of increased lumbar lordosis. Lumbar flexion and extension were moderately restricted. There was mild to moderate paravertebral tenderness maximal at the lumbosacral junction. Lower extremity neurological examination was normal.
Imaging findings with lumbar computerized tomography (CT) scan disclosed L5/S1 disk desiccation associated with moderate severity end-plate degenerative changes. Single-photon emission computerized tomography (SPECT) co-registered whole-body bone scan disclosed scintigraphic abnormality localised to the region of the L5-S1 disk, consistent with an active disk degenerative process. Lumbar magnetic resonance imaging (MRI) (Figure 1) disclosed substantial bone and degenerative disk change at L5/S1 with a reduction in disk space height and end-plate signal abnormality (Modic type 1). Mild broad-based disk bulging with relatively minor thecal and nerve root encroachment as well as mild to moderate bilateral foraminal narrowing compressing distal L5 nerve root sheaths. The remaining disks were normal.
Figure 1. Pre-BVNA Saggital MRI Images, Demonstrating Modic Type 1 Endplate Changes at the L5 Inferior End-plate and S1 Superior End-plate
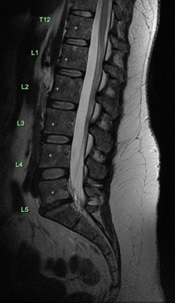
a) T2 weighted
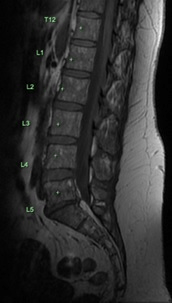
b) T1 weighted
Bilateral L5/S1 facet joint injections with bupivacaine and dexamethasone were provided and gave 50% pain reduction. Therefore, RFA of the L4 medial branch and L5 dorsal ramus was provided bilaterally, but with only mild benefit.
BVNA was then provided with the bipedicular placement of radiofrequency electrodes (a technique described below).
Description of a Technique of Lumbar Vertebrectomy via Pediculotomy and Basivertebral Nerve Ablation with Bipolar Radiofrequency Electrodes
The patient is positioned comfortably in the prone position with positioning aids to optimise lumbar lordosis. Sedation anesthesia and monitoring, maintaining responsiveness to voice, and intravenous antibiotic prophylaxis is provided.
Multiplanar fluoroscopy views are obtained as follows.
1. Anatomical anteroposterior (AP) view – identify the juncture of the superior articular process and transverse process at each target.
2. Trajectory view – optimise the pedicle shadow with slight cranial and ipsi-oblique rotation (typically 3-5°).
3. Lateral view – for dorsal to ventral depth.
4. AP view (+/- craniocaudal adjustment) – for a pseudo-axial view of the pedicle and to avoid medial pedicle breach.
In the trajectory view, the skin and deep tissues are infiltrated with preservative-free 0.5% Bupivacaine starting over the 1:00 o’clock (right) or 11 o’clock (left) position of the pedicle via a 25-gauge spinal needle advanced to the target. A stab incision is made to the skin with a number 11 scalpel blade, and then a 101 mm 11-gauge needle (T-handle Jamshidi needle; BD, Vernon Hills, IL, USA) is gently advanced to the pedicle at a point midway between the superior border of the superior articular process (SAP) and the location of the mamillo-accessory notch. Gentle taps with a surgical mallet position the needle firmly into the dorsal pedicle. The needle is then gently driven about halfway (1/2) from dorsal to ventral in the pedicle in the lateral view.
In the AP +/- decline view, the needle in the pedicle is confirmed to avoid the pedicle’s medial wall so as to avoid any medial breach. The AP view is re-checked as required. The needle is gently advanced further in the lateral view until a release on exit from the dense pedicle matrix and entry to the intra-vertebral trabecular bone. The needle is then further advanced within the vertebral body to about one-quarter (1/4) of the distance from dorsal to ventral in the lateral view.
The needle/stylet and trocar thus perform a vertebrectomy of the trabecular bone and create a path for the radiofrequency electrode – bone marrow aspiration aids in cavity creation. The trocar is advanced with multiple passes while remaining in the dorsal two-thirds of the vertebral body for each pass.
In the trajectory view, the lumen of the needle is identified. The needle is gently removed, and the hypolucency in the pedicle shadow left by the needle is identified. The radiofrequency electrode (Nimbus 17-gauge multi-tined expandable electrode; Stratus Medical, Magnolia, TX, USA) is placed through the pedicle hole, and the tip is positioned medially within the vertebral body spanning the mid-portion of the vertebral body in the lateral view.
A second radiofrequency electrode is placed using the same technique through the contralateral pedicle, optimally positioned in the vertebral body and precisely superimposed in angle and degree of insertion (Figure 2).
Figure 2. Flouroscopy Sequence at L5
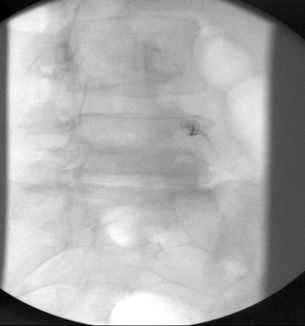
a) Trajectory view, crisp superior end-plate, ipsilateral oblique tilt, 25 G needle on dorsal aspect of right pedicle, approximately 1 o’clock
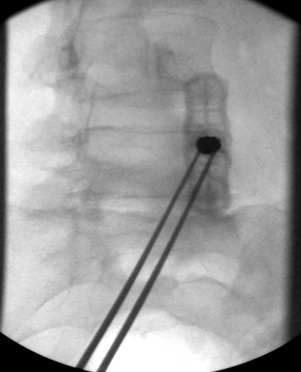
b) Trajectory view, 11G needle on the dorsal aspect of the pedicle
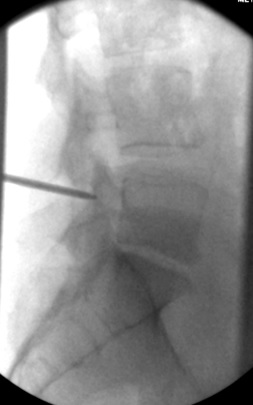
c) Lateral view, 11G needle engaging dorsal aspect of pedicle
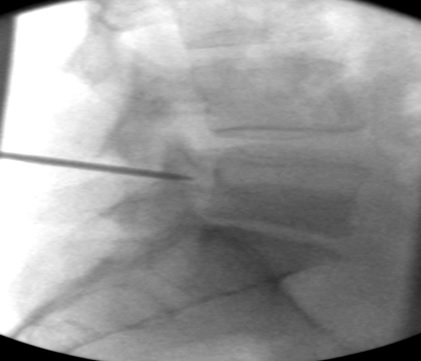
d) Lateral view, 11G needle, mid-pedicle
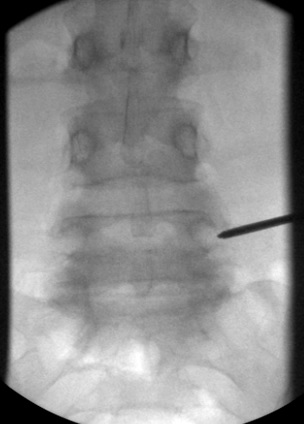
e) AP view, 11 G needle engaged in the mid-pedicle, note clear of medial wall of pedicle
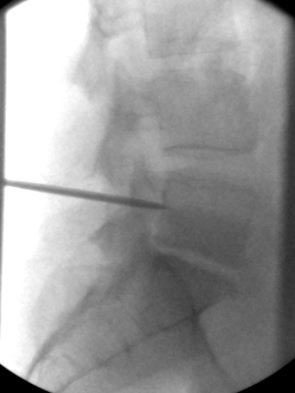
f) Lateral view, 11G needle at ventral aspect of the pedicle
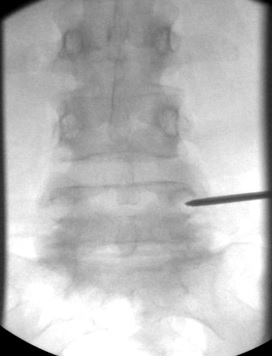
g) AP view, 11G needle at ventral aspect of the pedicle
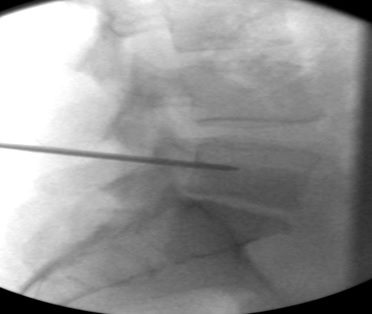
h) Lateral view, 11G needle within the vertebral body
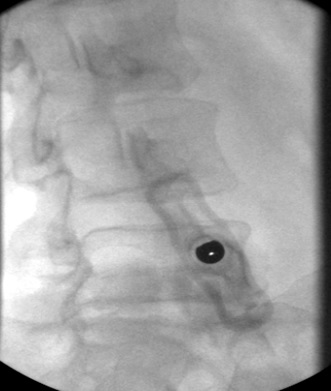
i) Trajectory view, prior to removing 11G needle
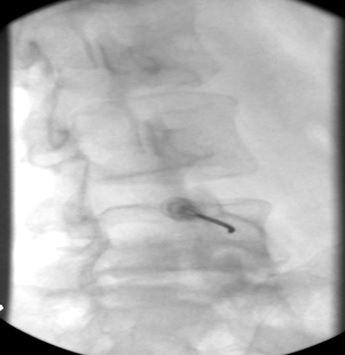
j) Trajectory view, 17G radiofrequency electrode at entry to pedicle hole
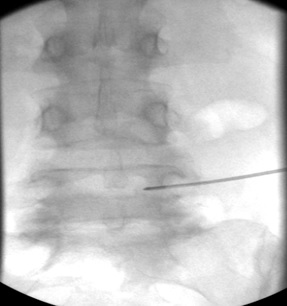
k) AP view, 17G radiofrequency electrode within vertebral body
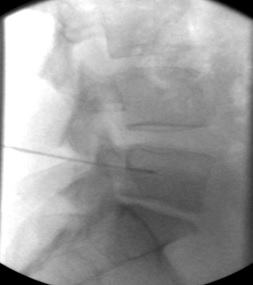
l) Lateral view, 17G radiofrequency electrode within vertebral body
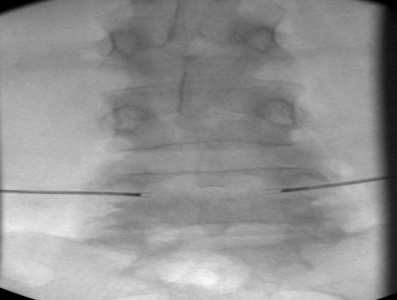
m) AP view, after repeating procedure on the left side to place 17 G radiofrequncy electrodes within vertebral body
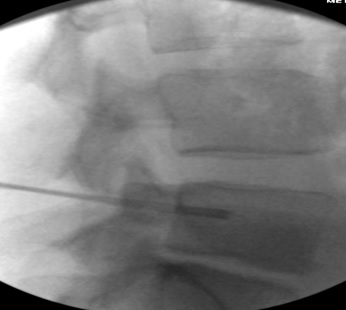
n) Lateral view, 17G radiofrequency electrodes in position for bipolar lesion within the vertebral body, note that the tines are deployed
A bipolar lesion is provided at 80° for four minutes following a 60-second ramp from 50 to 80 °C. The anodal temperature reaches 70 to 75 °C, indicating technical success for this aspect of the procedure.
The scalpel incision is closed with a 1-inch Steristrip, and a sterile dressing is applied.
RESULTS
The procedure was well-tolerated. There was moderate post-procedure discomfort reported for a few weeks.
Outcomes data were collected at six weeks using the following psychometric instruments: NRS, brief pain inventory (BPI), ODI, depression anxiety and stress scale (DASS21) and the EQ-5D-5L.
Lumbar MRI (Figure 3) demonstrated new sclerosis with surrounding bone marrow oedema of the right and left sides of the L5 and S1 vertebral bodies consistent with the BVNA treatment.
Figure 3. 6-weeks Post-BVNA MRI Images, Demonstrating New Sclerosis Consistent with BVNA Lesions
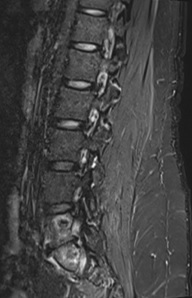
a) Right para-saggital STIR image
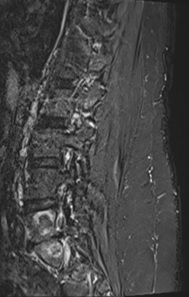
b) Left para-saggital STIR image
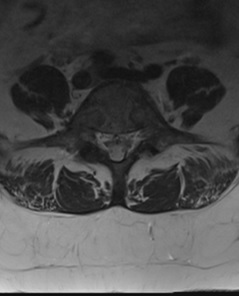
c) Axial T2 weighted image at L5
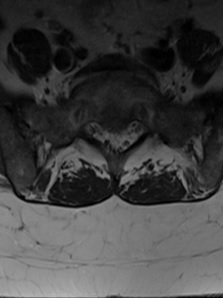
d) Axial T2 weighted image at S1
Oxycodone dose was decreased (40 mg daily) with a weaning plan.
Pain severity at six weeks post-BVNA was rated as averaging 3/10 over the previous week using the NRS (Table 1).
| Table 1. Psychometric Data Pre-BVNA and 6-Weeks Post-BVNA |
| Measure |
Pre-BVNA |
6-week post-BVNA |
| NRS |
8 |
3 |
| BPI-Severity |
32 |
15 |
| BPI-Interference |
70 |
23 |
| ODI (%) |
57 |
35 |
| ODI-Category |
Severe |
Moderate |
| DASS21-Depression |
28 (extremely severe) |
16 (moderate) |
| DASS21-Anxiety |
24 (extremely severe) |
8 (mild) |
| DASS21-Stress |
24 (moderate) |
18 (mild) |
| EQ-5D-5L (health state) |
43444 |
32332 |
| EQ-5D-5L (VAS) |
30 |
50 |
DISCUSSION AND CONCLUSION
BVNA is a promising technique to treat vertebrogenic pain. The industry sponsored the published randomized controlled trials4,5,6,7 performed using the Intracept device (Relievant Medsystems, Minneapolis, MN, USA) and this is a potential source of systematic bias.1 This case reports technically successful BVNA using the bipedicular placement of radiofrequency electrodes, including positive early outcomes data. The early result is consistent with the published literature for the uni-pedicular approach using the Intracept device. No industry funding was sought or received.
Precise knowledge of the anatomy and care need to be taken with pedicular access so as to avoid medial pedicle breach, to achieve adequate placement such that the bipolar lesion incorporates the precise location of the basivertebral nerve, and to avoid heating neural structures. Sedation anesthesia maintaining responsiveness to voice is thought to be an important additional safety consideration.
The clinical decision making process in this case was to first exclude the facet joints as a source of significant nociception and although facet intra-articular injections were moderately positive, the radiofrequency ablation of the target facets provided minimal benefit. BVNA was then provided with significant benefit.
Longer-term data collection is required to document sustainability of outcome with the bi-pedicular technique. A case series is envisaged to document outcomes in a community practice setting.
Ideally, independent studies of outcomes will help elucidate the long-term outcomes of BVNA, and refute or validate the technique.
ACKNOWLEDGEMENT
Dr Robert Wright provided the concept of using the bi-pedicular bipolar RFA approach.
CONSENT
The authors have received written informed consent from the patient.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.

























