INTRODUCTION
The impact of Sleep-Disordered Breathing (SDB) on behavioral outcomes and neurocognitive functions has received growing attention in the last decade.1 SDB is often viewed as a spectrum, with Primary Snoring (PS, i.e. snoring without apnea, hypoventilation or sleep fragmentation) being at the mild end, and Obstructive Sleep Apnea (OSA, i.e. various degrees of hypoxemia, hypercapnia, and sleep fragmentation) at the most severe end.2,3 Snoring is a primary and major clinical symptom in both categories. Snoring occurs in children of all ages, but snoring frequency is higher among preschool-aged children than among older children.4 The prevalence of snoring in children ranges from 5-12%, while approximately 1-4% have OSA.5
There is evidence that childhood snoring is associated with parent-reported behavioral problems of both externalizing and internalizing nature.6 The strongest associations for externalizing behaviors include hyperactivity, impulsivity, emotional lability, delinquency, conduct problems, aggressive behavior, and oppositional behavior.7,8,9,10,11,12,13,14 Snoring children have also internalizing problems, showing more anxious/depressed mood, somatic complaints, withdrawal, thought problems, and social problems.10,11,12,13,15,16,17,18 A few studies have used teacher reports,6,7,19 showing that teachers report substantially fewer problems than parents. In a study by Ali, et al.20 teachers estimated that the children in high risk group of sleep and breathing disorders were more hyperactive and inattentive than the controls. On the other hand, Arman, et al.7 found no significant differences in behavioral scales at school setting between the two groups. Kohler, et al.6 found poor agreement between parent and teacher reports of individual child behavior.
Previous studies have reported on the significant associations between childhood snoring and a diffuse pattern of impairments in neurocognitive functions. Most studies report significant differences between snoring and non-snoring children in intelligence, attention, and executive functions.15,17,19,21,22,23 Less commonly reported deficits are in memory, visual-spatial ability, language skills, and sensomotor functions.9,17 Despite these differences, it is notable that the mean Intelligence Quotient (IQ) and subtest scores for snoring children have usually been within the standardized normal range.22 Poor academic performance has been found in snoring children,8 as well as higher risk for academic underachievement even after snoring has resolved.24 In a longitudinal study, children with a history of SDB in the first 5 years of life had increased likelihood of having special educational needs at the age of 8.25 To our knowledge, studies widely investigating the association between snoring and both behavioral and neurocognitive implications are sparse.9,10,15,17,20
The focus of the present study was in school-aged children and the aim was to assess the behavioral problems and neurocognitive functioning in snoring and non-snoring children. Based on previous studies it was hypothesized that school-aged snoring children have elevated scores on problem behavior.7,8,11,13,16 In addition, it was hypothesized that snoring school-aged children perform worse than non-snoring peers in neurocognitive functioning, showing a diffuse pattern with various mild impairments in attention, executive function, verbal and global intelligence, and memory.21,26
METHODS
Participants
This study is a part of a larger study evaluating sleep and sleep-related disorders in school-aged children. The larger study consisted of a sample of 1538 6- to 10-year-old children in Tampere, Finland. Seventeen primary schools of a total 32 located in the city of Tampere were randomly selected. Three primary schools for deaf, motor skill disordered, specific language skill disordered, and mentally handicapped children were excluded. Parents of children enrolling in the first- or third-grade classes in selected schools received a questionnaire asking the demographics and background data and the Finnish version of the Sleep Disturbance Scale for Children (SDSC), developed and validated by Bruni, et al.27 SDSC is an instrument for assessing the frequency of sleep problems and snoring in school-aged children. The questionnaire was handed in physical examination by the school nurse or at class by the teacher. The sleep questionnaire included a question about snoring: “How often does your child snore?” The child was classified as snorer, if the parent answered the child to snore “often” (3 to 5 nights a week) or “always” (every night). Non-snoring children snored according to their parents “never” or “occasionally” (1 to 2 nights a month).
A total of 831 questionnaires were given to the first-graders and 190 were returned (23%). The third-graders received 707 questionnaires and 101 were returned (14%). Five children were excluded from the research data; four because of missing information about snoring prevalence rate, and one who was no longer in the first grade. Thus, the analyses included 186 first-graders and 100 third-graders (=286 children). Finally, 62 parents had expressed willingness to participate in the clinical part of the study and their children had overnight Polysomnographic (PSG) assessment and neurocognitive tests at the Sleep Laboratory in Tampere University Hospital. All the parents gave their informed written consent. The study was approved by the Ethical Committee of Tampere University Hospital and the City of Tampere.
Measurements of Behavioral Problems and Neurocognitive Functioning
Problem behaviors were identified using well-validated and internationally widely used assessment tools. Behavioral problems were assessed using the Problem Scales of the Child Behavior Checklist (CBCL, parent version)28 and equivalent Teacher Report Form (TRF, teacher version), both for 6- to 18-year-old children.28 The questionnaires have 113 questions and yield 8 scales: Anxious/Depressed, Withdrawn/Depressed, Somatic Complaints (these three constitute index for Internalizing Problems), Social Problems, Thought Problems, Attention Problems, Rule-Breaking Behavior, Aggressive Behavior (last two constitute index for Externalizing Problems), and Total Problems. For description of the data, the raw scores of the CBCL and TRF are individually converted into T-scores. Scales have a mean T-score of 50 and a standard deviation of 10. The borderline is T=65-69, and the clinical range is T>69. The borderline range for Internalizing, Externalizing and Total Problems is T=60-63 and clinical range T>63. In this study the reliability (Cronbach alpha) for the CBCL was .945 and for the TRF .952.
The Conners’ Parent Rating Scale-Revised (CPRS-R)29 and the Conners’ Teacher Rating Scale-Revised (CTRS-R),29 both for 3- to 17-year-old children, were used to identify behavioral problems as well. The CPRS-R has 80 questions and yields 7 scales: Oppositional, Cognitive Problems/Inattention, Hyperactivity, Anxious-Shy, Perfectionism, Social Problems, and Psychosomatic and the indices Attention Deficit Hyperactivity Disorder (ADHD), Restless-Impulsive, Emotional Lability, Total Index, DSM-IV Inattentive, DSM-IV Hyperactive-Impulsive, and DSM-IV Total. The CTRS-R has 59 questions and yields same scales excluding Psychosomatic and has all the same indices. For description of the data, the raw scores of the CPRS-R and CTRS-R are individually converted into T-scores. The CPRS-R and CTRS-R scales and indices have a mean T-score of 50 and a standard deviation of 10. The borderline T=56-60, and T-scores of 65 and above indicate a clinically significant problem. In this study the reliability (Cronbach alpha) for the CPRS-R was .964 and for the CTRS-R .959.
Parents completed their questionnaires at the Sleep Laboratory and teachers at school. On average, teachers reported to have taught the child for nine months, but the range was quite broad; from two months to 36 months. One child was excluded from the TRF analyses because the teacher had known her only for one month. Achenbach & Rescorla28 suggest that the TRF can be used when a teacher has known a child for at least two months.
Neurocognitive functions were assessed with standardized tests. The tests were chosen to measure both intellectual functioning and specific neurocognitive functions in five domains. Children’s intellectual functioning was evaluated using the Finnish version of the Wechsler Intelligence Scale for Children (WISC-III).30 Scores for Verbal Intelligence Quotient, Performance Intelligence Quotient, and Full Scale Intelligence Quotient were estimated by the following six subtests: Information, Similarities, Arithmetic, Picture Completion, Block Design, and Object Assembly. The intelligence quotients have a mean of 100(SD=15).
To evaluate specific neurocognitive functions the Developmental Neuropsychological Assessment (NEPSY, Finnish version) was used.31 The NEPSY subtests were chosen to obtain a comprehensive assessment of each domain for this age group. The five age-appropriate domains and their subtests were: Attention and Executive Function (Tower, Auditory Attention and Response Set, Visual Attention), Language Function (Phonological Processing, Comprehension of Instructions, Speeded Naming), Sensomotor Function (Fingertip Tapping, Imitating Hand Positions, Visuomotor Precision), Visuospatial Function (Design Copying, Arrows), and Memory and Learning Function (Memory for Faces, Memory for Names, Narrative Memory). The domains have a mean of 10(SD=3). For description of the data, the raw scores of the WISC-III and the NEPSY were individually converted into standard scores.
All children underwent a three hour neurocognitive evaluation (with one break) at the Sleep Laboratory in Tampere University Hospital. A trained psychologist or a trained psychology student administered the standardized tests individually to each child. The examiner was unaware whether the child was a snorer or a non-snoring one.
Statistical Analysis
Analysis were done using the Statistical Package for the Social Sciences (SPSS) software (Version 18.0 for Mac OS X). Due to limited number of participants and skewed distributions, the nonparametric Mann-Whitney U-test was used to test for group differences in behavioral and neurocognitive parameters. Chi square analyses were used to test for group differences in socio-economic variables. P-values <0.05 were considered statistically significant.
RESULTS
Demographics and Background Data
The demographics and background data of the participants are presented in Table 1. The data consisted of 27 snoring children (11 girls and 16 boys) and their 35 non-snoring peers (17 girls and 18 boys). There were no significant differences for age, gender, asthma, tonsillectomy or adenoidectomy. The groups did not differ with respect to support received at school. No significant differences were found in parental educational status or parental smoking. The snoring children had significantly higher Body Mass Index (BMI) than the non-snoring peers (U=312.00, p=.023).
| Table 1: Demographics and background data for snoring and non-snoring children. |
|
Snoring Children(N=27) |
Non-snoring Children(N=35) |
P-value |
| Mean age(SD) |
7.8(1.1) |
8.1(1.1) |
ns |
| Mean body mass index(SD) |
18.6(3.4) |
16.7(2.4) |
.023* |
|
n(%) |
n(%) |
|
| Gender |
|
|
ns |
| Girls |
11(40.7) |
17(48.6) |
|
| Boys |
16(59.3) |
18(51.4) |
|
| Part-time special education or remedial teaching |
7(25.9) |
7(20.0) |
ns |
| Asthma |
3(11.1) |
3(8.6) |
ns |
| Tonsillectomy |
3(11.1) |
2(5.7) |
ns |
| Adenoidectomy |
9(33.3) |
9(25.7) |
ns |
| Maternal education |
|
|
ns |
| Basic |
0(0) |
2(5.7) |
|
| Vocational training |
12(44.4) |
15(42.9) |
|
| High school |
3(11.1) |
4(11.4) |
|
| Polytechnic |
3(11.1) |
2(5.7) |
|
| University |
6(22.2) |
11(31.4) |
|
| Unknown |
3(11.1) |
1(2.9) |
|
| Paternal education |
|
|
ns |
| Basic |
3(11.1) |
3(8.6) |
|
| Vocational training |
9(33.3) |
10(28.6) |
|
| High school |
2(7.4) |
3(8.6) |
|
| Polytechnic |
2(7.4) |
4(11.4) |
|
| University |
8(29.6) |
14(40.0) |
|
| Unknown |
3(11.1) |
1(2.9) |
|
| Parental smoking |
|
|
ns |
| Mother |
6(22.2) |
7(20.0) |
|
| Father |
4(14.8) |
7(20.0) |
|
| Unknown |
6(22.2) |
6(17.1) |
|
On average, according to parents, both snoring and non-snoring children slept 9.7 hours per night. Parents reported that five (19%) of the snoring children snored every night and 22(81%) snored 3 to 5 nights a week. In the non-snoring group 18(51%) children never snored and 17(49%) snored occasionally (1 to 2 nights a month).
Behavioral Problems
As a group, snoring children had significantly more problems than non-snoring children on several CBCL and CPRS-R subscales. Figure 1 presents the main results of the CBCL and the TRF. When measured with the CBCL, internalizing problems and total problems were significantly more prevalent in the snoring group. On the Internalizing scale, 12 children (44%) of the snoring group and only one child in the non-snoring group scored on the clinical range. Snoring children also had statistically higher scores than non-snoring children on the following CBCL subscales: Anxious/Depressed, Withdrawn/Depressed and Thought Problems. No significant difference was found between the groups in the amount of externalizing problems.
Figure 1: Scores on the Child Behavior Checklist and Teacher Report Form for snoring and non-snoring children. 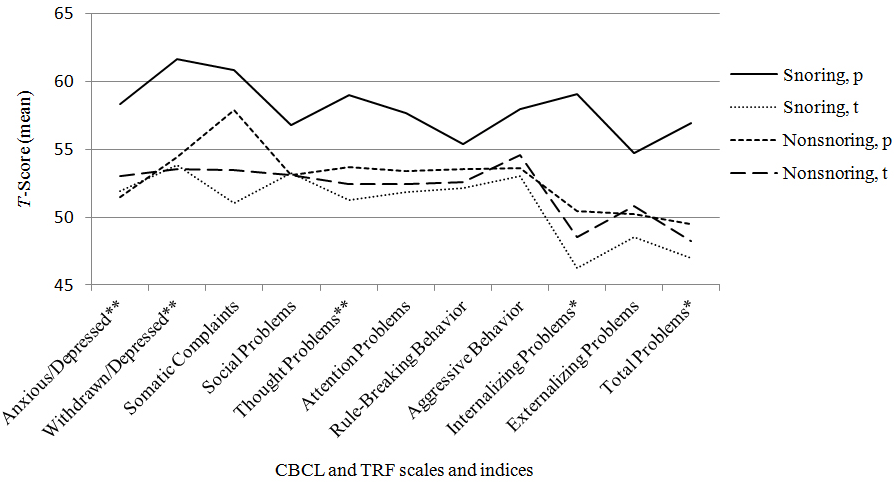
p=parent, t=teache; p<.05. **p< .01.
The teachers did not report many significant behavioral problems in the TRF in snoring children. Besides this, the teachers rated snoring children with lower scores in all scales and indices than the parents did. This tendency was not as evident with non-snoring children. Teachers estimated in the TRF that non-snoring children had significantly more somatic symptoms than snoring children (U=254.00, p=.025).
Figure 2 shows the results of the CPRS-R and the CTRS-R. Based on the CPRS-R scores, snoring children were significantly more anxious and shy and had more social problems and psychosomatic symptoms than non-snoring peers (Figure 2). Twelve snoring (44%) and 6 non-snoring (18%) children scored on the borderline or clinical range on the Psychosomatic scale, 11 snoring (41%) and 3 non-snoring (9%) children on the Social Problems scale and 9 snoring (33%) and 4 non-snoring (12%) children on the Anxious-Shy scale. The teachers did not report many significant behavioral problems in the CTRS-R in snoring children. In the CTRS-R, the teachers estimated non-snoring children to be significantly more impulsive and hyperactive than snoring children (U=231.00, p=.042). Although there was a lot of variability in how long the teacher had taught the child (from two months to 36 months), no statistically significant differences were found between snoring and non-snoring groups in these times.
Figure 2: Scores on the Conners’ Parent Rating Scale-Revised (CPRS-R) and Conners’ Teacher Rating Scale-Revised (CTRS-R)
for snoring and non-snoring children.
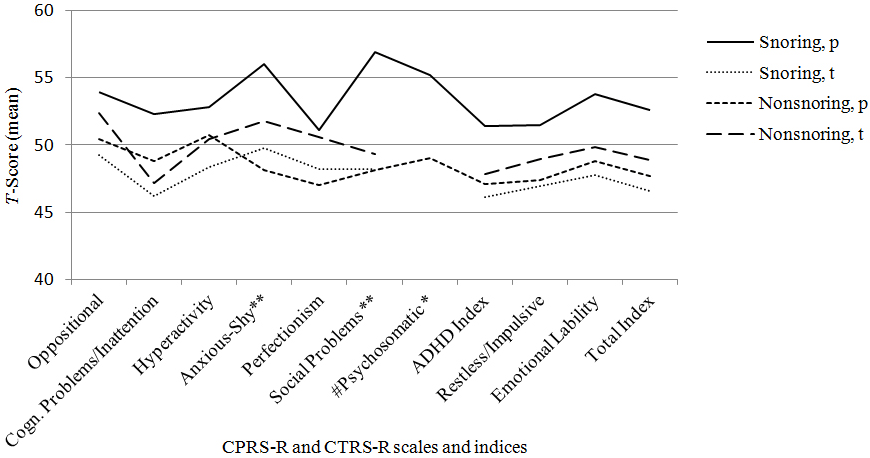
p=parent, t=teacher #=not evaluated by teachers; *p<.05. **p<.01.
Neurocognitive Functions
Table 2 summarizes the results of the neurocognitive functions. On average, intellectual functioning was within normal range for both groups. Although snoring children had lower scores than non-snoring peers on each WISC-III subtests and in Verbal, Performance and Full Scale IQ scores, these differences were not statistically significant. Similarly, the performance for both groups in the NEPSY subtests was within normal range. There were no significant differences between the groups in any of the fourteen subtests or in the five neurocognitive domains.
| Table 2: Mean Standard scores, standard deviations, and ranges of the WISC-III intelligence scores and NEPSY domains for snoring and non-snoring children. |
|
Snoring children (N=27) Mean±SD (Range) |
Non-snoring children (N=35) Mean±SD (Range) |
P-value
Mann-Whitney
U-test
|
| WISC-III |
|
|
|
| Intelligence |
|
|
|
| Verbal |
101.6±16.0(73-131) |
107.3±16.9(73-156) |
.221 |
| Performance |
99.4±17.4(64-136) |
106.3±18.6(71-132) |
.123 |
| Full scale |
100.2±14.8(75-124) |
105.9±16.4(76-141) |
.164 |
| NEPSY |
|
|
|
| Domains |
|
|
|
| Attention and Executive Function |
11.4±2.0(8-16) |
11.4±2.0(7-15) |
.870 |
| Language Function |
10.5±2.0(6-15) |
10.5±2.5(6-14) |
.943 |
| Sensomotor Function |
10.4±1.9(5-14) |
10.4±1.6(7-13) |
.609 |
| Visuospatial Function |
10.2±2.4(5-14) |
10.8±2.3(5-15) |
.354 |
| Memory and Learning |
9.9±2.6(3-14) |
9.9±2.1(6-13) |
.837 |
DISCUSSION
The aim of this study was to describe behavioral problems and neurocognitive functioning in snoring and non-snoring school-aged children. Previously, only a few studies have widely investigated both behavioral problems and neurocognitive functions in snoring school-aged children.
Firstly, as expected, the findings in this study indicate that snoring children have more parent-reported internalizing behavioral problems (including anxious, depressed, withdrawn, and psychosomatic symptoms), thought problems, social problems, and total problems than their non-snoring peers. Moreover, snoring children not only had higher incidence of internalizing problems, the problems also were more severe, showing that almost half of the children had clinically significant symptoms. These findings are consistent with existing SDB studies showing that children with snoring have more behavioral problems than their non-snoring peers, particularly symptoms of withdrawn, depressed and anxious mood, somatic complaints, social problems, and thought problems.16,17 Similar findings have been reported in younger children; Aronen, et al15 found in a Finnish preschool group (aged 3-6 years) that snoring children had significantly more internalizing symptoms, especially anxious and depressed mood and emotional reactivity than non-snoring peers. Despite these parent-reported behavior problems, teachers in this study did not report snoring children to have more behavioral problems than non-snoring peers. Teachers rated children with lower scores than parents.6,7,19
Hypothesis concerning elevated externalizing behavioral problems was not confirmed; in contrast to previous studies,11 current results do not support the association between snoring and externalizing behavioral problems, especially hyperactivity, oppositional and aggressive behavior in snoring children. In this study, externalizing symptoms were no more frequent in snorers than non-snorers.
Secondly, on the basis of previous studies,20,21,26 it was hypothesized that diffuse neurocognitive impairments would be present in children with snoring. Although, analyses showed that snorers had lower scores in the intellectual functions, these differences were not statistically significant. In addition, snorers’ intelligence quotients were within the normal range. This means that contrary to initial hypothesis, in neurocognitive measurements there were no significant differences between the two groups. All studied children attended mainstream schools and received only part-time special education or remedial teaching, which may partially explain these results. There were no children with observed learning disabilities among participants.
In this study it was considered important to obtain teachers’ observations on children’s behavior in the school settings, because objective measures in naturalistic settings other than parents have been sparse and results have been controversial. The inconsistency between parent and teacher reports of behavior was evident in this study, especially in the snoring group. This is consistent with previous reports showing that parents and teachers perceive the same children quite differently.7,29,32 The difference in parent and teacher reports in this study brings some possible explanations into mind. First, there was some variation in how long the teacher had known the child. Teachers with the lowest knowing time might have been cautious in reporting internalizing behavioral problems. Second, in the classroom children with internalizing problems are less visible than children with impulsive-hyperactive problems or aggressiveness. Therefore, teachers may have had difficulties in recognizing anxious, depressed, and shy children in the class. Thirdly, poor agreement between parents and teachers may also reflect the fact that children behave differently at home and in school environment. At home they are more likely to show emotional difficulties to their parents and at school demands for behavior are different.
There are several strengths in the present study. This study has the advantage of using simultaneously parent- and teacher-reported data, age-appropriate control group and standardized tests of neurocognitive functions and widely used and well-validated rating scales. Participants in this study were recruited from the local mainstream schools and were not being evaluated for sleep disturbances, behavioral problems or neurocognitive problems. The questionnaires were handed to all children, so the original sample of 1538 children was representative, and it can be thought that the participants in this study represent Finnish pupils attending mainstream schools. The data consisted only school children aged 6-10, so the age range is very limited compared to many other SDB studies.
The current study has also limitations. The data on both snoring and behavioral problems relied on parental reports. Reliance on parental perceptions may introduce potential measurement error. It has been suggested33 that parental-report questionnaires of children’s snoring can be used as substitute predictors of snoring. In studies by O’Brien, et al17 and Blunden, et al.19 parent-reported snoring was confirmed by polysomnography. Taken together, parents seem to be anapplicable source of information regarding their children’s snoring. Because the categorization of the children into snoring and nonsnoring groups in this study was based on parental reported snoring, it was not possible to examine association between snoring severity and behavioral or neurocognitive impairments.
The participation percent was quite low, probably due to inconvenience of the study protocol. During the PSG studies, the children (and most parents) slept two successive nights at the sleep laboratory and at the day of the psychological assessment children were absent from school. For some families this may have been too demanding of a procedure and therefore they chose not to participate in the clinical part of the study. Rather than having age and gender matched controls, we chose to use non-snoring participants as controls. Matching the two groups precisely to age and gender would have been too time-consuming and difficult, especially given the small number of participants.
The cause of observed behavior problems cannot be answered by this study. The beginning or duration of snoring was not examined. Studies investigated pediatric snoring longitudinally are needed to clarify this issue. Potential mechanisms of these problems in snoring are unknown and need further investigation.
In conclusion, the results of this study indicate that compared with non-snoring peers, school-aged snoring children are at risk for internalizing problems, thought problems, and social problems. Children who have sleep-related symptoms should be referred to diagnostic testing and possibly treatment. Also children with daytime somnolence, problems with behavior, or school performance, should have their sleep evaluated. Although there were no neurocognitive problems to be shown between the two groups in this study, internalizing problems, if not treated, may cause severe consequences in the long run, leading to social isolation, severe psychological difficulties, learning problems, and poor school performance.
ACKNOWLEDGMENT
This study was financially supported by Competitive State Research Financing of the Expert Responsibility area of Tampere University Hospital (Grants 9P013, 9R007, 9S007).
CONFLICTS OF INTEREST
The authors certify that there is no conflict of interest regarding the material discussed in the manuscript.







