INTRODUCTION
The metabolic diseases and especially the most frequent ones, type 2 diabetes mellitus (DM) and metabolic syndrome affect both genders.1,2,3,4 Several studies were conducted to assess the relationship between the gender and end-organ damage of diabetes mellitus.5,6,7,8,9,10,11,12,13 Previous studies investigated different organ systems and cardiovascular risk profiles, lipid profiles, risk of diabetic foot, risk factors for malignancy, and bone changes were evaluated in regard to gender difference in diabetic population.6,7,8,9,10,11,12 Diabetic retinopathy (DR) is an important and devastating end-organ damage of DM which affects approximately one fifth of the patients after 20 years of disease duration.14 Diabetic macular edema (DME) is the most frequent cause of visual loss in the patents with DR.15,16,17 Intravitreal anti-vascular endothelial growth factor (anti-VEGF) agents are the most frequently preferred agents in the treatment of DME. Patients who were treated earlier with anti-VEGFs are likely to have better treatment outcomes.17 In our daily practice, we realized that women with DME generally admitted later and with lower visual acuity than men.Therefore, in this study we aimed to compare the baseline visual and anatomical parameters between the female and male DME patients who underwent intravitreal ranibizumabtreatment.
METHODS
In this retrospective case-control study, medical records of the patients who had DME and underwent intravitreal ranibizumab (IVR) treatment between January 2013 and December 2015 in Istanbul Beyoglu Eye Training and Research Hospital were analyzed. Treatment naïve DME patients who were newly diagnosed and completed a follow time of 12 months in our clinic were included. The patients who were lost to follow-up in the first 12 months during our follow-up were not included. A written informed consent was obtained from all patients before the treatment. The study adhered to the tenets of the Declaration of Helsinki.
Data collected from the patients’ records included age, gender, best corrected visual acuity (BCVA), central retinal thickness (CRT), and intraocular pressure (IOP) at the baseline, and at month 3, 6, 9, and 12. Visit and injection numbers during the first 12 months were also recorded. Patients were divided into two groups according to their genders and compared in regard to baseline and other parameters. Also the patients were divided into three groups in regards to baseline visual acuity and the gender groups were compared in regard to distribution of the patients into this three groups. Group 1 consisted of the patients with a baseline BCVA ≤1.0 logarithm of the minimum angle of resolution (LogMAR), group 2 consisted of the patients between 0.9 and 0.4 LogMAR, and group 3 consisted of the patients with a BCVA ≥0.3 LogMAR.
All patients underwent a standardized examination including measurement of BCVA via a projection chart at 4 meters, slit-lamp biomicroscopy, measurement of IOP via applanation tonometry, and biomicroscopic fundus examination. Fundus photography, fluorescein angiography (FA) (HRA-2; Heidelberg Engineering, Heidelberg, Germany), and optical coherence tomography (OCT) imaging (Spectralis; Heidelberg Engineering, Heidelberg, Germany) were performed before treatment. All examinations were repeated monthly, except for FA. Fluorescein angiography was repeated according to the physicians’ discretion. Optical coherence tomography was used for detecting macular edema and measurement of CRT. Central retinal thickness, defined as the mean thickness of the neurosensory retina in a central 1 mm diameter area, was computed using OCT mapping software generated by the device. Diabetic macular edema was diagnosed via FA and OCT,and patients with a CRT of >300 microns were considered to have DME. The severity of DR, angiographic classification of DME, and ischemic status of macula were not assessed.
All injections were performed under sterile conditions after application of topical anesthesia, use of 10% povidone-iodine (Betadine; Purdue Pharma, Stamford, CT, USA) scrub was used on the lids and lashes, and 5% povidone-iodine was administered on the conjunctival sac. Intravitreal ranibizumab 0.5 mg/0.05 ml (Lucentis; Novartis, Basel, Switzerland) was injected through the pars plana at 3.5 mm posterior to the limbus with a 30 -gauge needle. Patients were instructed to admit back the hospital if they experienced decreased vision, eye pain, or any new arising symptoms.
Initially, all of the patients were prescribed to receive a loading dose of three consecutive monthly injections. Then the patients wereplanned to befollowed monthly, and a single injection of IVR was repeated when the BCVA decreased by one or more lines, or an increase of >100 microns in CRT in OCT images compared to the last visit, or disappearance of foveal pit compared to the last visit.
Primary outcome measures of this study were the baseline BCVA and CRT in the female and male patients. Secondary outcome measures were the change in BCVA and CRT during the 12 months follow-up period.
Statistical Analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) software (version 20.0). Visual acuity was converted to the LogMAR for statistical analysis. Categorical variables were presented as numbers and percentages, while numerical variables were expressed as the mean and standard deviation. First the data was analyzed in terms of normality using Kolmogorov-Smirnov test. As the distribution of the data was found to be normal, the visual acuity and the CRT values between baseline and the other time points were assessed with repeated measures test. The means within the groups were compared using independent sample t-test. Categorical variables were compared using chi-square test. A p value <0.05 was considered statistically significant.
RESULTS
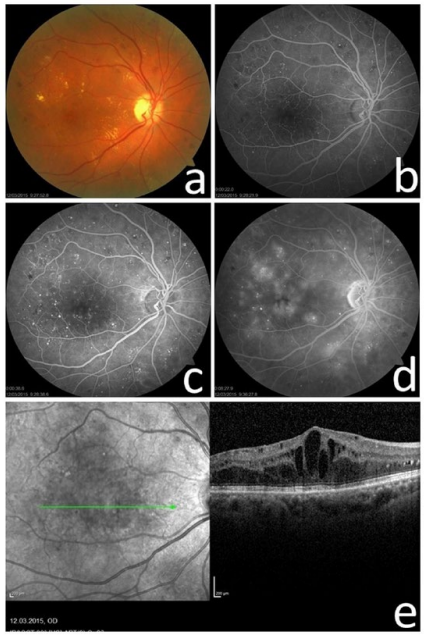
All of the included patients were Caucasians. Sixty-three eyes of 44 women, and 110 eyes of 76 men were included in the study. The mean age of the women were 59.2±8.2 (range 36-74 years), and men were 56.9±9.1 (range 26-79 years) (p=0.2). The other baseline characteristics were similar between the two groups (Table 1) (Figures 1 and 2).
Figure 1: a) Fundus Photograph, b,c,d) Fluorescein Angiograms, and e) Optical Coherence Tomography Scan of a Women with Diffuse DME with a Baseline Visual Acuity of 1.3 LogMAR.
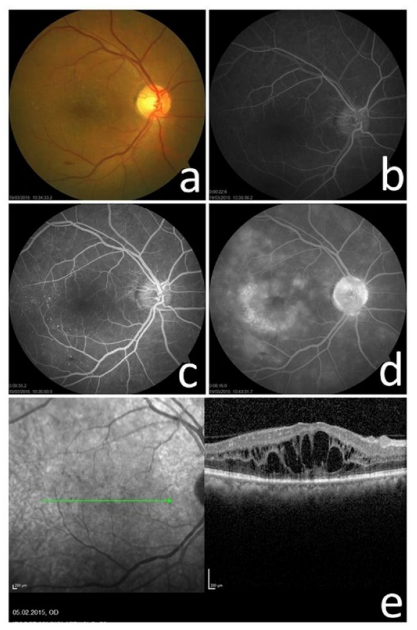
Figure 2: a) Fundus Photograph, b,c,d) Fluorescein Angiograms, and e) Optical Coherence Tomography Scan of a Men with Focal DME with a Baseline Visual Acuity of 0.5 LogMAR.
| Table 1: General Characteristics of the Patients. |
|
Women
|
Men |
p value
|
| Number of patients/eyes |
44/63
|
76/110 |
–
|
| Age (years) |
59.2±8.2
|
56.9±9.1 |
0.2
|
| Lens status (phakic/pseudophakic) |
45/18
|
83/27 |
0.5
|
| Baseline BCVA (LogMAR) |
0.72±0.44
|
0.49±0.31 |
<0.0001
|
| Baselne CRT (microns) |
484±117
|
467±100 |
0.3
|
| BCVA: Best Corrected Visual Acuiy; LogMAR: Logarithm of the Minimum Angle of Resolution; CRT: Central Retinal Thickness. |
The mean baseline BCVA of the women was 0.72±0.44 (range 0.1-2.0) LogMAR, and the men was 0.49±0.31 (range 0.0-2.0) which was statistically significant between the two groups (p<0.0001). In the women group 34.9% of the eyes (22 of 63) had a BCVA ≤1.0 LogMAR, whereas only 10.9% of the eyes (12 of 110) in the men group had a BCVA ≤1.0 LogMAR. The percentage of the patients with a visual acuity between 0.9 and 0.4 LogMAR was similar between the two groups and was 47.6% in the women and 52.7% in the men, respectively. The eyes with a BCVA ≥0.3 LogMAR compromised 17.5% (11 of the 63 eyes) of the women, and 36.4% (40 of the 110 eyes) of the men. The distribution of the BCVA groups between the two groups were statistically significant (p<0.0001). The mean BCVA levels of the two groups during the study period were summarized in Table 2. The change in mean BCVA was not statistically different between the two groups at any of the time points (p=0.4 for month 3, p=0.6 for month 6, p=0.6 for month 9, and p=0.08 for month 12, respectively).
The mean baseline CRT of the women was 484±117 microns (range 312-759), and the men was 467±100 microns (range 320-704) (p=0.3). The mean CRT levels of the two groups during the study period were summarized in table 2. The change in mean CRT was not statistically different between the two groups at any of the time points (p=0.9 for month 3, p=0.3 for month 6, p=0.3 for month 9, and p=0.2 for month 12, respectively).
| Table 2. LogMAR Visual Acuity Values and CRT Findings in Microns in the Phakic and Pseudophakic Groups at Different Time Points. |
|
Variables
|
Women |
Men |
| Baseline visual acuity, mean |
0.72±0.44 LogMAR (range 0.1-2.0) |
0.49±0.31 LogMAR (range 0.0-2.0)
|
|
Month 3 visual acuity, mean
|
0.60±0.45 LogMAR (range 0.0-2.0) |
0.46±0.38 LogMAR (range 0.0-2.0) |
| Month 6 visual acuity, mean |
0.59±0.45 LogMAR (range 0.1-2.0) |
0.39±0.30 LogMAR (range 0.0-1.3)
|
|
Month 9 visual acuity, mean
|
0.63±0.43 LogMAR (range 0.1-2.0) |
0.37±0.32 LogMAR (range 0.0-1.3) |
| Month 12 visual acuity, mean |
0.56±0.37 LogMAR (range 0.0-2.0) |
0.42±0.32 LogMAR (range 0.0-1.3)
|
|
Baseline CRT, mean
|
484±117 μ (range 312-759) |
467±100 μ (range 320-704) |
| Month 3 CRT, mean |
394±113 µ (range 216-624) |
392±108 µ (range 240-677)
|
|
Month 6 CRT, mean
|
364±116 µ (range 226-754) |
366±82 µ (range 228-600) |
| Month 9 CRT, mean |
403±154 µ (range 225-818) |
355±88 µ (range 227-707)
|
|
Month 12 CRT, mean
|
359±127 µ (range 199-741) |
373±109 µ (range 234-775)
|
| CRT: Central Retinal Thickness; LogMAR: Logarithm of the Minimum Angle of Resolution; vs.: Versus; μ: Micrometers. |
The mean visit number in women and men groups were 4.6±0.9 and 4.5±1.0, respectively (p=0.5). The mean injection number in women and men groups were 4.1±1.6 and 3.8±1.4, respectively (p=0.2).
DISCUSSION
In this study we focused on an interesting topic which was the baseline BCVA levels of DME patients in regard to gender. Women seemed to admit to ophthalmologists with worse mean baseline visual acuity levels than men revealed by this study. The mean baseline BCVA of women was found to be 0.72 LogMAR and men was 0.49 LogMAR. The difference between the gender groups was 2.3 LogMAR lines. However, the mean baseline CRTand the other parameters during the follow-op period was similar between the two groups. The change in mean BCVA and CRT, along with the mean visit and injection numbers did not differ significantly. Different previous studies evaluated the relationship between the gender and other coexisting diseases with DM.5,6,7,8,9,10,11,12 In a study by Al-Salameh et al, the association between gender and control of diabetes and other cardiovascular risk factors in patients with type 2 DM were investigated.6 In the study it was revealed that women were less likely to be smokers or ex-smokers, and less likely to have cardiovascular disease at the baseline than men. During the follow-up period of 3 years mean hemoglobin A1C levels and blood pressure measurements were similar between the two genders, only low-density lipoprotein (LDL) cholesterol levels were wound to be significantly higher in women. Madsen et al, evaluated the gender differences in cardiovascular risk profiles of ischemic stroke patients with diabetes.7 Of the assessed 3515 patients 1146 (33%) had DM. Among the several interpreted factors, no gender difference was found in patients with DM. However, it was reported that women with DM had strokes at a younger age. Self-efficacy in in diabetes self-management in type 1 DM patients was evaluated in a Danish population by Lindkvist et al.11 The self-efficacy levels was assessed with a questionnaire between the adolescent girls and boys and the authors reported that the relationship between self-efficacy and age seemed to differ between girls and boys. Gamboa et al, evaluated the race and gender differences in statin use and LDL cholesterol control among the DM patients.8 The recruited the patients with a LDL level >100 mg/dL, or were taking statins. In the study the authors divided the included patients into four groups which were white women, black women, white men, and black men, respectively. They reported that statin use was more frequent and LDL control was better in white women than the other three groups. Navarro-Paternella et al, conducted a cross-sectional trial regarding the differences between genders in relation to the factors which were associated with risk of diabetic foot in diabetic population.9 They included 174 patients without a history of stroke or systemic vascular disease.
In the study, it was reported that the risk factors associated with the development of diabetic foot were presented differently in female and male DM patients. The risk factors were reported to be older age, presence of calluses, and claw toes among women with DM, and were insulin use, presence of sensory comorbidities, ulcers, numbness, and stiffness in the feet among men. Dabrowski et al, evaluated the differences in risk factors of malignancy between men and women with type 2 DM in a retrospective multicenter study.10 They reported that metformin use was associated with lower cancer risk in both genders. Breast and uterine cancer was the most prevalent malignancy among women and obesity and insulin treatment was associated with increased risk of cancer. Colorectal and prostate cancer was the most prevalent cancer among men. Patsch et al, investigated the interactions between gender and type 2 DM in regard to morphology of peripheral skeleton.12 They concluded that skeletal hypertrophy was frequent in patients with DM, present in both genders with DM, and appeared attenuated at the tibial cortex in men. The gender differences in diabetic eye disease is assessed in two studies from Japan.5,13 Kajiwara et al reported that among Japanese patients with DM, females exhibited a significantly higher prevalence of proliferative DR than men at baseline. Therefore they concluded that female gender was an independent risk factor for the development of DR.5 In fact this study has a similarity to ours. Our women DME patients presented with lower BCVA levels than men and the women patients included in the study by Kajiwara et al more frequently presented with proliferative DR than the included men. The two findings, to present with more frequently with proliferative DR and with a lower visual acuity a baseline might both address a late admittance of women. We do not agree with the idea that female gender was accepted as an independent risk factor for the development of DR, because this might be solely secondary to the late admittance of women.In another study by Kamoi et al, risk factors for DME was evaluated and gender was not found to be associated with the risk of DME.13
In summary, interestingly diabetic women and men were not found to differ in regards to several discussed systemic disorders above. Also the control of diabetes and the prevalence of DR is known to be similar in both genders.1,2,3,4,5 However, the baseline visual acuity levels were found to be significantly difference between women and men in our study. In addition the clinical course was similar and the change in mean BCVA and CRT was similar.
LIMITATIONS
The main limitation of this study was its retrospective design and relatively low sample size for this kind of study. However, this is the first study indicating a difference between women and men in regard to baseline parameters and reporting treatment outcomes of DME. Also all of the included patents were treatment naïve and first time admitters, which were other two strong properties of the study.
CONCLUSION
In conclusion, our results simply revealed that there may be a significant difference in baseline BCVA between women and men with DME at the first admittance. We suppose that women with DME admit to ophthalmologist lately than men, or at least they wait for a more prominent visual decrease. Perhaps we should be more sensitive in periodic ophthalmology consultations of women with DME.
ACKNOWLEDGEMENTS
The study was in adherence to the Declaration of Helsinki, and consent forms were obtained from all of the patients before each treatment applied in the study. None of the authors have conflict of interest. All authors meet the requirements for authorship including substantive contributions, review and approval of the submitted work, and accept public responsibility for the final manuscript.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
FUNDING
This retrospective study was not supported by any company. None of the authors has financial or proprietary interests in any material or method mentioned. This data has not been previously published.







