INTRODUCTION
The National Osteoporosis Foundation has estimated that more than 100 million people worldwide are at a risk of developing fractures secondary to osteoporosis. Osteoporotic vertebral compression fractures (OVCFs) constitute a major healthcare problem in the Western Countries, not only because of the high incidence of these lesions, but also due to their direct and indirect negative implications on the patient’s quality of life (QoL) owing to health concerns, and the costs associated with the healthcare system. Compression fractures lead to a loss in the height of the vertebral segment, and the resulting spinal deformity can lead to a decrease in the pulmonary capacity and mobility, malnutrition and depression. Kyphosis secondary to the osteoporotic vertebral compression fractures attributes a 2 to 3 times greater incidence of death primarily on account of pulmonary causes.1,2,3,4
Although, the usual treatment strategy for an osteoporotic vertebral compression fracture may be bedrest, the application of analgesics, and bracing, some fractures may lead to progressive deformity and a debilitating pain. Several techniques have been developed for simpler and safer procedures during the last 2 decades. Techniques of vertebral body augmentation have been developed in an effort to treat these refractory cases. The high-pressure injection of low-viscosity poly-methylemethacrylate (PMMA) poses potential risks for neural compromise and pulmonary embolism due to uncontrolled leakage. Therefore, balloon kyphoplasty (BKP) and vertebroplasty, using a large cannula low-pressure injection of PMMA in a high viscosity state has been introduced.
Percutaneous kyphoplasty (PKP) is a recently developed, minimally invasive surgical approach for the treatment for OVCF. PKP with acrylic cement PMMA is a procedure aimed at preventing vertebral body collapse and pain in patients with pathological vertebral bodies. PKP is a promising therapeutic technique for controlling pain in patients with bone marrow failure. PKP for OVCFs are typically performed by delivering double balloons implementing a bilateral or unilateral transpedicular approach; the balloons are inflated simultaneously for elevating the end plate to support vertebral body height balanced restoration.
The deformity is purportedly corrected by the insertion and expansion of a balloon into a fractured vertebral body. After a reduction of the fracture bone, the cement is deposited into the cavity created by the balloon to repair the fracture. Good clinical outcomes as well as restoration of the vertebral body height have been reported with kyphoplasti.5,6,7
Absolute contraindications to any vertebral augmentation procedure includes asymptomatic osteoporotic vertebral compression fractures, local or systemic infection, uncorrectable coagulopathy, or improving pain with the use of appropriate medications. Fracture of the posterior elements (without vertebral body fractures) is among the other absolute contraindications of the procedure. The complications associated with balloon kyphoplasty include non-target PMMA embolization, adjacent vertebral body or rib fracture and infection.8
PATIENTS AND METHODS
A prospective analysis of 30 patients treated with kyphoplasty was performed at our institution. These patients had VCFs at levels T7 to L4 due to osteoporosis arising from primary and secondary osteoporosis. There were 41 VCFs in these 30 patients. The goals of this study were to determine the safety and effectiveness of kyphoplasty in improving vertebral body height, decreasing pain, and improving function. The study included 30 patients (10 male, 20 female), who were followed up after six months. The median age was 69 years (range 53-87 years). Subjects were excluded if they had associated spinal stenosis, neurologic deficit, an active infection, and severe comorbidities, such as uncorrected coagulopathy.
The date of the fracture was carefully determined by a retrospective chart review. During the pre-operative visit, the patient was subjectively asked to recall a traumatic event or the cause of the sudden onset of pain. The interval between the date of fracture and the date of surgery determined the age of the fracture. The age of the fracture could be determined for 39 of the 41 fractures based on this method of data collection. Two patients (no. 8, MS) and (no. 21, NM) could not recall a specific incident which marked the beginning of their focal pain. A fracture was defined as fresh up to the 28th day following the event causing the injury.
Physical examination combined with lateral radiographs, magnetic resonance imaging (MRI) and computerized tomography (CT), were performed to diagnose the vertebral body compression fractures.
Radiographic Evaluation
Standing films were used to detect the kyphosis of fractures associated with the vertebral body. In some patients, pre-operative standing films could not be obtained on account of reported pain. In these cases, measurements were taken from the available supine films (Tables 1 and 2).
| Table 1. Study Demographics |
| (Present & past), physical examination and neurological examination parameters |
Number |
| Subjects |
|
| No. Females |
20 |
| No. Males |
10 |
| Total number |
30 |
| Median subject age (years) |
69 |
| Range of age |
(53-87) |
| Etiology of index fracture |
|
| No. of primary osteoporosis cases |
27 |
| No. of secondary osteoporosis cases |
3 |
| Index fracture age |
|
| No. less than 60 |
3 |
| No. between 60-80 |
24 |
| No. more than 80 |
3 |
| Index facture treated |
|
| No. One |
21 |
| No. Two |
7 |
| No. Three |
2 |
| Location of fracture |
|
| Total number (T7-L4) |
41 |
| Thoracic (T7-T9) |
1 |
| Thoraco-Lumbar junction (T10-LV2) |
33 |
| Lumbar (LV3-LV4) |
7 |
| Table 2. Data Relevant to the Treated Patients: Initials, Age of the Patient, Sex, Level Treated, Age of the Fracture (Pre-operative Symptoms), Pre-operative Vertebral Kyphosis, Post-operative Vertebral Kyphosis |
| No. |
Patient |
Age |
Gender |
Age of fracture (days) |
Levelof fracture |
Anesthesia |
Pre-operative Kyphosis (°) |
Post-operative kyphosis |
Approach |
| 1 |
M.A |
76 |
M |
13 |
D12 |
L |
32 |
25 |
BI |
| 2 |
M.S |
64 |
M |
9 |
D12 |
L |
12 |
4 |
BI |
| 3 |
L.E |
76 |
F |
21 |
D7 |
L |
29 |
22 |
BI |
| 4 |
S.A |
76 |
F |
16 |
L3 |
G |
48 |
34 |
BI |
| 5 |
M.H |
72 |
F |
8 |
D12 |
G |
15 |
7 |
UNI |
| 5 |
M.H |
72 |
F |
8 |
L2 |
G |
2 |
0 |
UNI |
| 5 |
M.H |
72 |
F |
8 |
L4 |
G |
25 |
22 |
UNI |
| 6 |
A.H |
84 |
M |
3 |
L2,3 |
L |
11 |
9 |
UNI |
| 7 |
T.M |
62 |
F |
25 |
L4 |
L |
35 |
35 |
BI |
| 8 |
M.S |
85 |
M |
UNKNOWN |
D11 |
L |
18 |
18 |
BI |
| 9 |
G.M |
71 |
F |
16 |
D12 |
G |
10 |
8 |
UNI |
| 9 |
G.M |
71 |
F |
16 |
L2 |
G |
16 |
10 |
UNI |
| 10 |
M.M |
72 |
M |
5 |
D12 |
G |
8 |
8 |
BI |
| 10 |
M.M |
72 |
M |
5 |
L2 |
G |
12 |
10 |
BI |
| 11 |
F.A |
58 |
F |
35 |
D12,L1 |
G |
40 |
29 |
UNI |
| 12 |
Z.H |
87 |
F |
5 |
D10,11,12 |
G |
60 |
43 |
BOT |
| 13 |
M.S |
65 |
F |
18 |
D10,11 |
G |
28 |
17 |
BI |
| 14 |
L.M |
66 |
F |
9 |
D12 |
L |
20 |
11 |
UNI |
| 15 |
G.A |
62 |
M |
18 |
D12 |
G |
30 |
16 |
BI |
| 16 |
S.S |
53 |
F |
1 |
L1 |
G |
25 |
8 |
BI |
| 17 |
A.M |
74 |
M |
2 |
D10 |
L |
11 |
5 |
UNI |
| 18 |
S.A |
77 |
F |
12 |
L3 |
L |
6 |
2 |
UNI |
| 19 |
Z.A |
79 |
M |
14 |
L1 |
G |
10 |
7 |
BI |
| 20 |
F.E |
73 |
F |
33 |
D12 |
L |
16 |
12 |
UNI |
| 20 |
F.E |
73 |
F |
33 |
L3 |
L |
20 |
15 |
UNI |
| 21 |
N.M |
66 |
F |
UNKNOWN |
D10,1 |
G |
21 |
18 |
UNI |
| 22 |
N.D |
59 |
F |
18 |
D12 |
G |
16 |
12 |
BI |
| 23 |
L.O |
67 |
F |
6 |
L4 |
G |
38 |
29 |
BI |
| 24 |
K.B |
73 |
F |
5 |
D12 |
L |
15 |
12 |
UNI |
| 25 |
M.K |
63 |
M |
4 |
D12 |
G |
28 |
18 |
BI |
| 26 |
M.S |
65 |
F |
10 |
L1 |
G |
8 |
5 |
BI |
| 27 |
D.L |
71 |
F |
9 |
L1 |
L |
20 |
14 |
UNI |
| 28 |
A.N |
64 |
M |
8 |
L1 |
G |
30 |
22 |
UNI |
| 29 |
K.T |
60 |
F |
6 |
L1 |
G |
8 |
5 |
UNI |
| 30 |
M.A |
64 |
F |
7 |
L1 |
G |
12 |
10 |
UNI |
Kyphotic angles across the fractured levels were measured using the Cobb technique: measurements were taken from the superior end plate of the vertebra from one level above the treated vertebra, to the inferior endplate of the vertebral body one level below the treated vertebra. When non-adjacent levels of the vertebrae were treated in a patient, separate Cobb angles were measured for each treated level. When adjacent level vertebral fractures were treated, a single Cobb angle measurement across the treated levels was performed. Thoracic and lumbar fractures were analyzed for alterations in the angles of kyphosis and lordosis, respectively. Plain radiographs were obtained pre-operatively, immediate post-operatively, after three months and after the last follow-up. This method has been shown to be the least variable and the most reliable technique in the measurement of fractures due to kyphosis.
Not all patients underwent MRI on account of their financial issues. In those patients who underwent MRI, the fractured levels which needed augmentation, showed the evidences of edema on T2-weighted images and short T1 inversion recovery sequences (STIR view) following MRI; hence, causing pain. These patients were therefore considered for treatment. CT scans were performed in patients who were at a risk of developing posterior wall fracture. Fluoroscopic and radiographic images were used to monitor the extravasation associated with all the treated vertebral levels. The operation was performed when painful thoracic or lumbar osteoporotic fractures were diagnosed without affecting the posterior vertebral edge.
Device Design
The kyphoplasty system consists of a balloon, of which one or two each are inserted into the vertebral body and simultaneously inflated with a contrast saline solution, under a pressure of up to (150-200) PSI, to symmetrically expand both the balloons. After the balloon expansion is sufficient and/or complete, the balloons are deflated and retrieved, leaving both the restored height, and PMMA cement injected into the cavities (Figure 1).
Figure 1. Kyphoplasty Balloon Set

Surgical Technique
A radiolucent table and 2 C-arm fluoroscopy machines, Mobile Digital C-Arm were used for performing kyphoplasty at our institute. The 2 fluoroscopy machines placed orthogonally across the radiolucent table allowed simultaneous viewing of the anteroposterior and lateral projections of the fractured level. This process helped to speed up the operation and minimize the risks of contamination during the operation. The patient was then taken to the operating room, where general anesthesia was administered in 18 procedures and local anesthesia with heavy sedation in 12 interventions.
The patient was carefully turned prone and all bony prominences were protected. To prevent infection, a pre-operative single shot of I.V dose of a third-generation cephalosporin was administered. Two fluoroscopy machines were then wheeled into the position, and the fractured level was centered in both the anteroposterior and lateral projections, before the patient was draped and prepared for the surgery. The fluoroscopy machines were also covered with appropriate sterile covers following which a 1 cm incision was made, lateral and superior to both the pedicles.
Under continuous fluoroscopic guidance, the guide wires were inserted into the superior outer pedicle quadrant using slight manual pressure and controlled blows from a hammer. The working sleeves over a side opening cannula were applied and the guide wires were removed. At this stage, any bone fragment that was extracted through the cannulae, was collected for biopsy and sent for histopathological examination. A drill was manually twisted into the vertebral body to create a tract for the balloon. The balloon catheters were inserted bilaterally or unilaterally and this was continued till the white indicator on the catheter shaft aligned with the top of the working sleeves and then the packaging wires were removed.
The prepared kyphoplasty inflation systems were connected bilaterally and the air bubbles were evacuated out of the catheters. According to the visual (fluoroscopic) and manometric parameters, the criteria for stopping the balloon inflation were: when the fracture reduced, when the balloon violated the confines of the bone, when the inflation pressure reached (150-200) psi, or when a maximum balloon volume was obtained.
The balloons were gradually deflated and removed through the working sleeves. PMMA cement corresponding to the combined volume of the inflated balloons was inserted bilaterally, using the side-opening cannulae. Each step was performed under fluoroscopic control.
The criteria for stopping the cement injection was the incidence of cement leakage anteriorly, posteriorly or across the disc spaces and when the injected volume showed a reading of 3 cc. Thus, the cannulae were pulled back slightly but kept in position until the cement hardened, then twisted to break off any connected cement from being left behind in the pedicles (Figures 2, 3, 4, 5, 6, 7, 8 and 9).
Figure 2. Diagram and X-ray with an Orthograde View Through the Ipsilateral Pedicle (So-called en Face or Pedicle View Image)
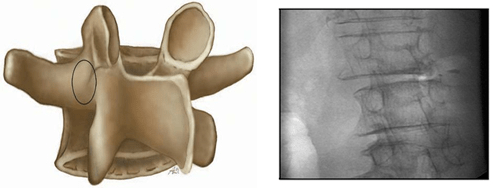
Figure 3. The Starting Point of Right Transpedicular Access between 1 and 3 o’clock.
The Skin Incision is 1 cm Lateral to this Point for L1 to L4, and 2 cm Lateral for L5
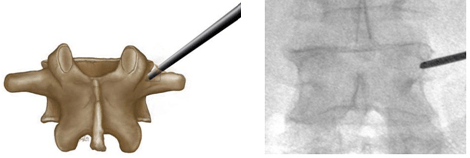
Figure 4. Starting Point of Left Transpedicular Access between 9 and 11 o’clock
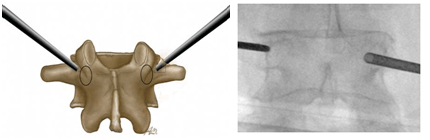
Figure 5. Ending Points for Transpedicular Access
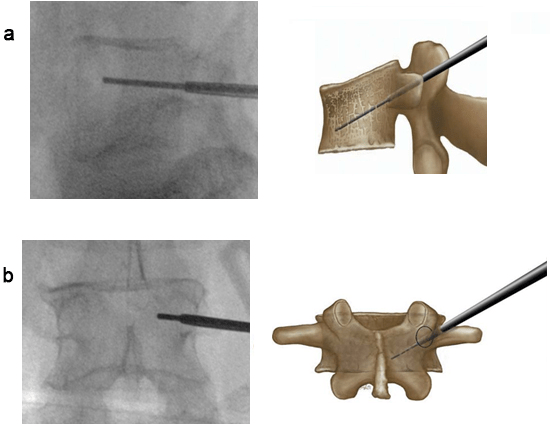
(a) Ending Point of the Kirschner wire on the Lateral Image Approximately 4 mm Dorsal to the Front Edge or
at the Transition Point 80%: 20% of the Vertebral Body. (b) Ending Point of the Kirschner wire on the AP Image
Figure 6. Introduction of the Jamshidi Needle and the Operating Cannula (osteointroducer)
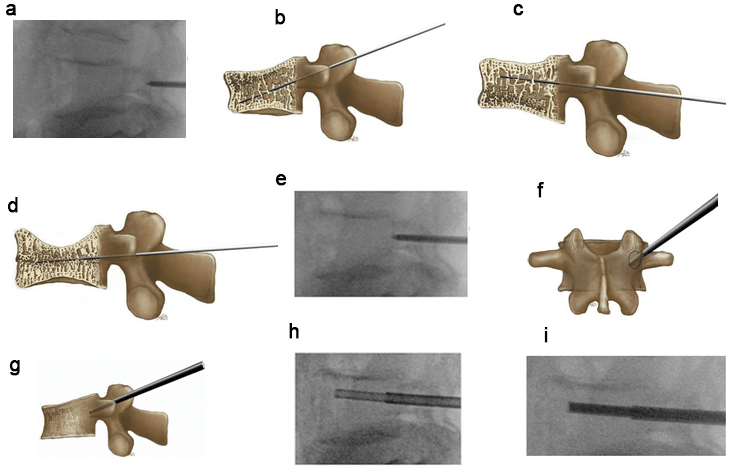
(a) Starting Point of the Jamshidi Needle on the Lateral View (b-d) The Cranial-Caudal Orientation of the Jamshidi Needle has to be
Adapted According to the Type of Fracture. (b) Fracture Type A1.2.1. (c) Fracture Type A1.2.3. (d) Fracture Type A1.3. (e) Ending Point of the Jamshidi Needle with a Slight Perforation of the Posterior wall on the Lateral View. (f) Ending Point of the Jamshidi Needle on the Anteroposterior View. The Medial Limitation of the Pedicle Ring should not be Exceeded. (g) Ending Point of the Operating Cannula Lying at Least 2-3 mm Ventrally of the Posterior wall of the Vertebral Body. (h) Biopsy with Bonefiller. (i) Preparing the Cannula for the Balloon
Figure 7. Non-Inflated Balloons in the Vertebra, both Markings of the Balloons must lie Outside the Operating Cannula.
Ending Points of the Balloons, the Balloons should not Perforate any Cortical Substance, Neither on the Antero Posterior, nor on the Lateral View.
The Removal of the Balloons Leaves an Extensive Cavity in the Vertebra
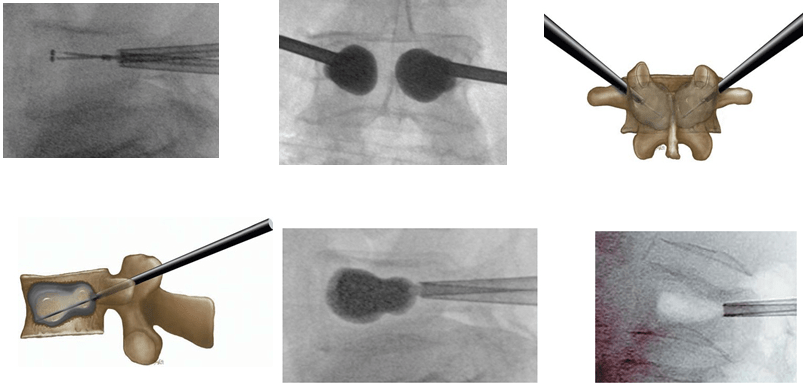
Figure 8. (a) Maximal Fracture Reduction Technique for a Patient with L1 Vertebral Fracture due to Osteoporosis. (a, d and i): Lateral View; (e, g and h): Frontal view. (b) Initial Balloon Inflation Leads to Partial Kyphosis Correction (Local Kyphosis Angle 9°). (c) Fracture Reduction Loss After Balloon Deflation (Local Kyphosis Angle 18°). (d) Reinflation of the 2 Balloons to Restore Maximum Fracture Reduction. (e and f): Deflation of only 1 balloon (e) and Cement injection on the same side (f). (g and h): After Polymethylmethacrylate Polymerization, Deflation of the Second Balloon (g) and Cement Injection on the same Side (h). (i) Final view showing Maintenance of the Initial Fracture Reduction (Local Kyphosis Angle 9)
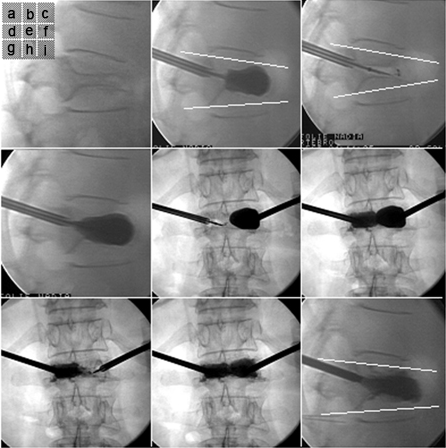
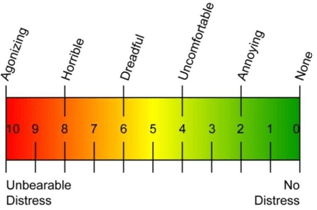
Figure 9. Visual Analog Scale (VAS)
Clinical Outcome Measurements
Clinical outcomes were measured pre-operatively and post-operatively using the visual analogue scale (VAS) and the qswestry disability index (ODI) score.
Visual Analog Scale (VAS)
Patients were asked to mark their pain on a scale of 0.0 to 10.0 cm where 0.0 cm indicated no pain at all and 10.0 cm was indicative of the worst pain imaginable. VAS scores were examined before the procedure, and 1, 4, 12 and 24 weeks after the procedure (Figure 9).
Oswestry Disability Index (ODI)
The ODI is a low back pain-specific questionnaire that assesses the ability of the patient to perform various activities of daily living. The ODI shows high test-retest reliability and is the most commonly recommended condition-specific outcome scoring measure in patients with chronic low back pain. Although, the ODI was initially developed specifically for low back pain, even thoracic fractures causing a great degree of referred low back pain and thus, impairing the functional capabilities of the affected individual, can be evaluated using the ODI score. The ODI scores were recorded before the procedure, and at 4, 12 and 24 weeks after the procedure.
RESULTS
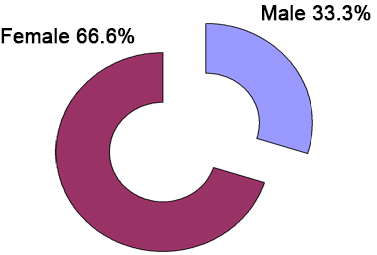
A total of 30 patients with 41 VCFs underwent cement augmentation at our institution from February 2011 to June 2013. The study population included 10 males (33.3%) and 20 females (66.6%). The median age of the patients was 69 years (range 53-87 years). The follow-up period was 24 weeks (Figure 10) (Table 3).
Figure 10. Study Population
| Table 3. Age Groups of the Patients |
| Age groups |
Number |
Percentage |
| Below age 60 years |
3 |
10 |
| 60-80 years |
24 |
80 |
| Above 80 years |
3 |
10 |
| Total |
30 |
100 |
There were 24 patients (80%) who were included in the age group (60-80 years), 3 patients (10%) who were included in the age group (above 80 years) and 3 patients (10%) who were aged less than 60-years-old. The mean age of the patients was 69 years (Table 4).
| Table 4. Mechanism of Injury |
| Mechanism |
Number |
Percentage |
| Simple fall |
14 |
46.7% |
| RTA |
6 |
20% |
| Lifting heavy |
4 |
13.3% |
| No obvious cause |
6 |
20% |
| Total |
30 |
100% |
The causes of injury were either simple fall on the ground for 14 patients (46.7%), real time adjudication (RTA) in 6 (20%) patients, 4(13.3%) patients had a history of lifting heavy objects preceding their feeling of pain and 6(20%) patients developed a sudden onset of pain without precipitating the incident (Table 5).
| Table 5. Index Fracture Age |
| Age of patients |
Number |
Percentage |
| No. less than 10 |
15 |
50% |
| No. 10-30 |
11 |
36.7% |
| No. 30-40 |
2 |
6.7% |
| Unknown |
2 |
6.7% |
| Total |
30 |
100% |
Fifteen (50%) patients presented with a history of fracture of less than 10 days whereas 11 (36.7%) patients reported the incidence of a fracture 10-30 days back. 2 patients (6.7%) presented with a history of fracture 30-40 days before. It was noted that in 2 patients (6.7%), the exact age of the fracture could not be estimated (Table 6).
| Table 6. Index Fracture Treated |
| Number of levels |
Number |
Percentage |
| No. One |
21 |
70% |
| No. Two |
7 |
23% |
| No. Three |
2 |
7% |
| Total |
30 |
100% |
In 21 (70%) patients, one level fracture was treated while in 7 patients (23%) two levels were augmented. In 2 (7%) patients, three levels were treated (Table 7).
| Table 7. Etiology of Fractures |
| Etiology |
Number |
Percentage |
| Primary |
27 |
90% |
| Secondary |
3 |
10% |
| Total |
30 |
100% |
There were 3 patients (10%) who reported secondary osteoporosis. This condition was caused by long-term corticosteroid therapy in one patient and renal failure in two patients. The remaining 27 (90%) patients were assumed to have primary osteoporosis (Figure 11).
Figure 11. Distribution of Fractures
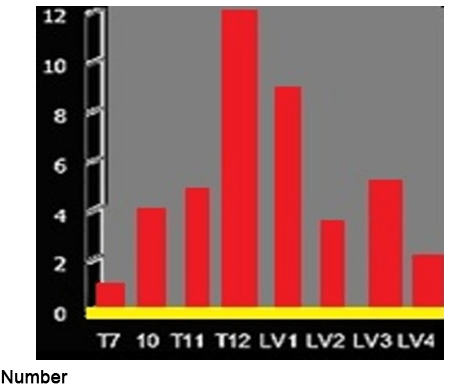
The largest concentration of fractures was detected in the thoraco-lumbar junction where T12 revealed 12 (27%) fractures, LV1 sustained 9 (30%) fractures and LV3 revealed 5 (11%) fractures (Table 8).
| Table 8. Mode of Anesthesia |
| Anesthesia |
Number |
Percentage |
| General |
18 |
60% |
| Local with heavy dose |
12 |
40% |
| Total |
30 |
100% |
Eighteen interventions were performed under intubation, and 12 (40%) patients were operated with the application of local anesthetics in combination with IV sedation (Table 9).
| Table 9. Post-operative Hospital Stay |
| Duration of stay |
Number |
Percentage |
| 1-3 days |
26 |
87% |
| 3-10 days |
4 |
13% |
| >10 days |
None |
None |
| Total |
30 |
100% |
All the patients were mobilized within the first 48 hours after surgery. 26 patients (87%) were hospitalized for 1-3 days. Due to additional injuries, 4 (13%) patients were kept in the hospital for a maximum of 10 days.
The Clinical Outcomes
Change in spinal sagittal alignment
The median kyphosis angle was 21.8° before the procedure and decreased by a median value of 6.7° after the operation. 60% of the patients experienced a reduction in kyphosis by more than 5° (Interquartile range of 10°). In an analysis of this subgroup of patients, faster pain relief was achieved relative to the patients who showed minimal to no reduction in the kyphotic angle. The VCFs were then analyzed in the spinal region (thoracic or lumbar). 23 thoracic vertebral compression fractures were treated in 18 patients with a median improvement of 5.8° (range 0° to 17°) in the sagittal alignment across the treated segments. 18 lumbar vertebral compression fractures were treated in 16 patients with a 5.6° (range 0°-17°) median improvement in the angle of lordosis (Table 15). If fractures that were considered reducible by BKP (defined as at least 5° improvement in sagittal alignment) were analyzed, 18 fractures in 18 patients showed an improvement in the sagittal alignment of 10° (range 6° to 17°) (Table 1). Statistical significance in improvement of the sagittal angle from pre-operative to postoperative measurements reached the p<0.0001 level (Wilcoxon signed-rank test) for all fractures combined, thoracic fractures, lumbar fractures, and reducible fractures (Tables 10, 11 and 12).
| Table 10. Data of the Treated Patients: Level Treated, Pre-operative Vertebral Kyphosis, Reduction Achieved, and Correction in Percent |
| No. |
Patient |
Level of Fracture |
Kyphotic
pre-operative (°) |
Kyphotic
post-operative (°) |
Reduction (°) |
Reduction (%) |
| 1 |
M.A |
D12 |
32 |
25 |
7 |
22% |
| 2 |
M.S |
D12 |
12 |
4 |
8 |
67% |
| 3 |
L.E |
D7 |
29 |
22 |
7 |
24% |
| 4 |
S.A |
L3 |
48 |
34 |
14 |
29% |
| 5 |
M.H |
D12 |
15 |
7 |
8 |
53% |
| 5 |
M.H |
L2 |
2 |
0 |
2 |
100% |
| 5 |
M.H |
L4 |
25 |
22 |
3 |
12% |
| 6 |
A.H |
L2,3 |
11 |
9 |
2 |
18% |
| 7 |
T.M |
L4 |
35 |
35 |
0 |
0% |
| 8 |
M.S |
D11 |
18 |
18 |
0 |
0% |
| 9 |
G.M |
D12 |
10 |
8 |
2 |
20% |
| 9 |
G.M |
L2 |
16 |
10 |
6 |
37% |
| 10 |
M.M |
D12 |
8 |
8 |
0 |
0% |
| 10 |
M.M |
L2 |
12 |
10 |
2 |
16% |
| 11 |
F.A |
D12,L1 |
40 |
29 |
11 |
27% |
| 12 |
Z.H |
D10,11,12 |
60 |
43 |
17 |
28% |
| 13 |
M.S |
D10,11 |
28 |
17 |
11 |
39% |
| 14 |
L.M |
D12 |
20 |
11 |
9 |
45% |
| 15 |
G.A |
D12 |
30 |
16 |
14 |
47% |
| 16 |
S.S |
L1 |
25 |
8 |
17 |
68% |
| 17 |
A.M |
D10 |
11 |
5 |
6 |
54% |
| 18 |
S.A |
L3 |
6 |
2 |
4 |
66% |
| 19 |
Z.A |
L1 |
10 |
7 |
3 |
30% |
| 20 |
F.E |
D12 |
16 |
12 |
4 |
25% |
| 20 |
F.E |
L3 |
20 |
15 |
5 |
25% |
| 21 |
N.M |
D10,11 |
21 |
18 |
3 |
14% |
| 22 |
N.D |
D12 |
16 |
12 |
4 |
25% |
| 23 |
L.O |
L4 |
38 |
29 |
9 |
23% |
| 24 |
K.B |
D12 |
15 |
12 |
3 |
20% |
| 25 |
M.K |
D12 |
28 |
18 |
10 |
36% |
| 26 |
M.S |
L1 |
8 |
5 |
3 |
38% |
| 27 |
D.L |
L1 |
20 |
14 |
6 |
30% |
| 28 |
A.N |
L1 |
30 |
22 |
8 |
27% |
| 29 |
K.T |
L1 |
8 |
5 |
3 |
38% |
| 30 |
M.A |
L1 |
12 |
10 |
2 |
17% |
| Table 11. Improvement in Spinal Sagittal Alignment |
|
N
(Fractures/Patients) |
Median
Preoperative Cobb Angle (Range) |
Median
Postoperative Cobb Angle (Range) |
Median
Change in CobbAngle (Range) |
p-value* |
| Overall |
41/30 |
21.8° (1-60°) |
16° (2-43°) |
6.7° (0-17°) |
˂0.0001 |
| Thoracic |
22/18 |
17.8° (8-60°) |
12.9° (4-43°) |
5.8° (0-17°) |
˂0.0001 |
| Lumbar |
19/16 |
19.8° (2-48°) |
14.8° (0-34°) |
5.6° (0-14°) |
˂0.0001 |
| Readily reduced° |
18/18 |
29° (11-60°) |
19° (4-43°) |
10° (6-17°) |
˂0.0001 |
* Wilcoxon signed-rank test, preoperative vs. postoperative Cobb angles.
° Improvement in kyphosis of >5°. |
| Table 12. Change in Kyphoticangle |
|
No. of patients |
Percentage |
| Minimal to reduction˂2 |
3 |
7% |
| Reduction 2-5 degrees |
17 |
42% |
| Reduction 6-10 degrees |
11 |
27% |
| Reduction 11-17 degrees |
10 |
24% |
| Total |
41 |
100% |
Note that, in 3 fractures (7%), the reduction was achieved by less than 2°, while a reduction of 2-5° occurred in about 17 fractures (42%). In 11 (27%) fractures, a reduction of about 6-10°. On the other hand, a reduction by about 11-17° was achieved in about 10 reported fractures.
Visual Analogue Scale (VAS)
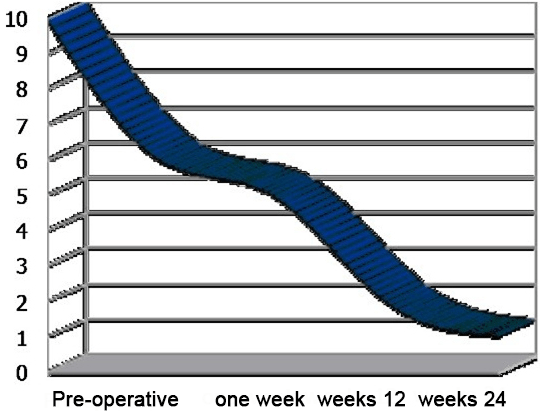
The median VAS scores dropped from 8.7 pre-operatively to 3.5 within one week post-operatively , to 1.7 at 12 weeks following the procedure and to 1.5 at 24 weeks and in the last follow-up (Figure 12) (Table 13).
Figure 12. Median VAS Pain Scores in which 1 Indicates No Pain and 10 Indicates Severe Pain. Compared with
Pre-operative Scores, there was a Significant Pain Relief (p˂0.001) at all Post-operative Values
| Table 13. Pain Score as given on VAS (1-10), Pre-operatively and at Follow-ups. For Average Values, 1 Patient Counts as a Single Procedure |
| No. |
Patient |
Level of fracture |
Pre-operative |
1 week |
12 weeks |
24 weeks |
| 1 |
M.A |
D12 |
6 |
2 |
2 |
0 |
| 2 |
M.S |
D12 |
8 |
6 |
0 |
2 |
| 3 |
L.E |
D7 |
9 |
8 |
8 |
9 |
| 4 |
S.A |
L3 |
10 |
6 |
5 |
2 |
| 5 |
M.H |
D12 |
8 |
2 |
2 |
1 |
| 6 |
A.H |
L2,3 |
10 |
6 |
5 |
4 |
| 7 |
T.M |
L4 |
10 |
0 |
0 |
1 |
| 8 |
M.S |
D11 |
8 |
6 |
3 |
1 |
| 9 |
G.M |
D12 |
8 |
2 |
0 |
0 |
| 10 |
M.M |
D12 |
7 |
1 |
4 |
5 |
| 11 |
F.A |
D12,L1 |
10 |
0 |
0 |
0 |
| 12 |
Z.H |
D10,11,12 |
6 |
4 |
4 |
1 |
| 13 |
M.S |
D10,11 |
10 |
2 |
– |
– |
| 14 |
L.M |
D12 |
7 |
1 |
0 |
1 |
| 15 |
G.A |
D12 |
10 |
3 |
0 |
0 |
| 16 |
S.S |
L1 |
9 |
4 |
1 |
0 |
| 17 |
A.M |
D10 |
8 |
3 |
1 |
0 |
| 18 |
S.A |
L3 |
10 |
8 |
7 |
4 |
| 19 |
Z.A |
L1 |
7 |
5 |
3 |
2 |
| 20 |
F.E |
L3 |
9 |
5 |
2 |
7 |
| 21 |
N.M |
D10,11 |
8 |
5 |
1 |
0 |
| 22 |
N.D |
D12 |
10 |
4 |
0 |
1 |
| 23 |
L.O |
L4 |
9 |
3 |
0 |
0 |
| 24 |
K.B |
D12 |
10 |
6 |
2 |
6 |
| 25 |
M.K |
D12 |
10 |
3 |
0 |
0 |
| 26 |
M.S |
L1 |
8 |
0 |
0 |
0 |
| 27 |
D.L |
L1 |
9 |
3 |
0 |
1 |
| 28 |
A.N |
L1 |
9 |
3 |
2 |
1 |
| 29 |
K.T |
L1 |
8 |
1 |
0 |
– |
| 30 |
M.A |
L1 |
9 |
3 |
– |
– |
Two patients reported deterioration; one of them (no. 20, F.E) indicating a change of score from “2” after a 3 months follow-up to“7” after 6 months. On further examination of this patient, no adjacent level fractures were detected and the patient was treated conservatively using epidural injections which improved the patient’s condition and physiotherapy and medications were continued. The other patient (no. 18, S.A) reported an exacerbating condition of pain, 6 months following the BKP. Upon further assessment of the patient, no adjacent level fractures were noted, but the patient’s condition presented a clinical picture of discitis for which the patient is still undergoing further investigation and treatment (Table 14).
| Table 14. Modes of Improvement |
|
Number |
Percentage |
| Immediate post-operative follow-up |
26 |
87% |
| Over 3 months |
2 |
2% |
| Constant |
24 |
80% |
| Further |
2 |
7% |
| Further |
7 |
23% |
26 (87%) patients achieved a dramatic post-operative improvement whereas, in 2 cases, significant improvement was reported after the 3 months follow-up. On the other hand, 2 (7%) patients showed a further deterioration in the pain score. 24 (80%) patients showed a constant improvement over a period of 6 months, with further improvement noted in 7 patients (23%) (Table 15) (Figure 13).
| Table 15: Oswestry Disability Index. |
|
Means±SE |
Median |
Std. Deviation |
Interquartile |
| Pre-operative ODI |
80± 2.5 |
85.000 |
14.529 |
22.500 |
| 1 month post-operative |
32± 3.256 |
32.6 |
13.200 |
18.200 |
| 3 months post-operative |
21.4667±2.909 |
22.000 |
10.936 |
13.500 |
| Last follow-up |
17.933±2.727 |
18.000 |
6.339 |
9.400 |
| SE: standard error; ODI: Oswestry Disability Index; Std. deviation: Standard deviation. |
Figure 13. Mean ODI Scores
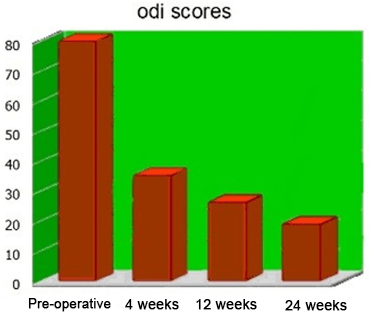
The mean pre-operative ODI score was 80, decreasing to 32 following the 1st month post-operatively, and improving to 21 and 17 after the 3-month and the last follow-up, respectively (Figure 14).
Figure 14. Median ODI Score
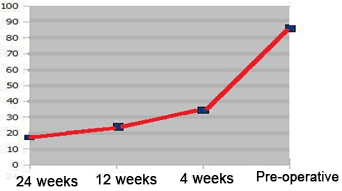
The median pre-operative ODI score was 85.00, improving to 32.6 after the 1st month post-operatively, and decreasing to 22 and 18 after the 3rd month and the last follow-up (Table 16).
| Table 16. ODI Scores, Pre-operatively and at Follow-ups. 1 Month, 3 Months and 6 Months Post-operative |
| No. |
Patient |
Level of fracture |
Pre-operative |
4 weeks |
12 weeks |
24 weeks |
| 1 |
M.A |
D12 |
60.00 |
20.00 |
36.00 |
24.00 |
| 2 |
M.S |
D12 |
56.00 |
2.00 |
2.00 |
24.00 |
| 3 |
L.E |
D7 |
80.00 |
52.00 |
80.00 |
74.00 |
| 4 |
S.A |
L3 |
94.00 |
72.00 |
72.00 |
64.00 |
| 5 |
M.H |
D12 |
82.00 |
34.00 |
20.00 |
20.00 |
| 6 |
A.H |
L2,3 |
96.00 |
70.00 |
64.00 |
64.00 |
| 7 |
T.M |
L4 |
98.00 |
2.00 |
2.00 |
2.00 |
| 8 |
M.S |
D11 |
66.00 |
70.00 |
38.00 |
42.00 |
| 9 |
G.M |
D12 |
60.00 |
6.00 |
2.00 |
2.00 |
| 10 |
M.M |
D12 |
72.00 |
2.00 |
34.00 |
26.00 |
| 11 |
F.A |
D12,L1 |
100.00 |
2.00 |
2.00 |
2.00 |
| 12 |
Z.H |
D10,11,12 |
76.00 |
56.00 |
58.00 |
26.00 |
| 13 |
M.S |
D10,11 |
100.00 |
8.00 |
– |
– |
| 14 |
L.M |
D12 |
52.00 |
2.00 |
0.00 |
0.00 |
| 15 |
G.A |
D12 |
88.00 |
20.00 |
2.00 |
0.00 |
| 16 |
S.S |
L1 |
100.00 |
28.00 |
0.00 |
0.00 |
| 17 |
A.M |
D10 |
80.00 |
18.00 |
0.00 |
0.00 |
| 18 |
S.A |
L3 |
100.00 |
90.00 |
76.00 |
42.00 |
| 19 |
Z.A |
L1 |
88.00 |
66.00 |
42.00 |
36.00 |
| 20 |
F.E |
L3 |
86.00 |
50.00 |
20.00 |
38.00 |
| 21 |
N.M |
D10,11 |
80.00 |
56.00 |
26.00 |
0.00 |
| 22 |
N.D |
D12 |
100.0 |
36.00 |
6.00 |
0.00 |
| 23 |
L.O |
L4 |
80.00 |
32.00 |
2.00 |
0.00 |
| 24 |
K.B |
D12 |
100.00 |
44.00 |
22.00 |
58.00 |
| 25 |
M.K |
D12 |
100.00 |
26.00 |
0.00 |
0.00 |
| 26 |
M.S |
L1 |
80.00 |
18.00 |
2.00 |
0.00 |
| 27 |
D.L |
L1 |
100.00 |
40.00 |
42.00 |
0.00 |
| 28 |
A.N |
L1 |
100.00 |
32.00 |
8.00 |
8.00 |
| 29 |
K.T |
L1 |
80.00 |
4.00 |
0.00 |
2.00 |
| 30 |
M.A |
L1 |
98.00 |
30.00 |
2.00 |
0.00 |
Complications
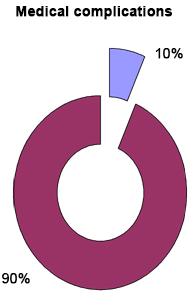
There were 3 medical complications reported. Seven days after BKP, pulmonary embolism was diagnosed in a patient (no. 8, M. S) with a history of deep vein thrombosis (no evidence of PMMA leakage to the lungs by (CT angiography). The other two patients (no. 1, M.A and no. 18, S.A) underwent peri-operative renal impairment marked by an increase in the creatinine level and thus needed close medical follow-up (Table 17) (Figure 15).
| Table 17: Medical Complications. |
|
Number |
Percentage |
| Medical complications |
3 |
10% |
| No medical complications |
27 |
90% |
| Total |
30 |
100% |
Figure 15. Shows that 10% of the Patients Developed Pre-operative Medical Complications
Cement Leakage
Evaluation of intraoperative and post-operative radiographs revealed extra vertebral cement leaks in 11 (27%) of the 41 vertebral fractures treated (Tables 18 and 19) (Figure 16).
| Table 18. Percentage of Cement Leakage |
|
Number |
Percentage |
| Cement leakage |
11 |
27% |
| No cement leakage |
30 |
73% |
| Total |
41 |
100% |
| Table 19. Location of cement leakage |
| Location of leakage |
Number |
Percentage |
Complications |
| Adjacent intervertebral |
3 |
27% |
No |
| Anterior to vertebral |
4 |
36% |
No |
| Lateral to vertebral body |
3 |
27% |
No |
| In the spinal canal |
1 |
9% |
No |
| Total |
11 |
100% |
No |
Figure 16. Percentage of Cement Leakageable 21 Locations of Leakage
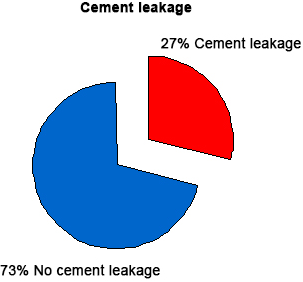
In 3 fractures, the cement leaked into the adjacent intervertebral disc, in 4 fractures, leakage occurred anterior to the vertebral body, and in 3 fractures the leakage occurred lateral to the vertebral body and in 1 patient, (no. 17, A.M) the cement leaked posteriorly into the spinal canal but with no obvious complications. All PMMA extravasations were asymptomatic, the cement remained in the immediate vicinity of the treated vertebrae, and no medical or surgical intervention was required to remove the extravasated PMMA. The clinical results of the group of patients with PMMA extravasation did not differ from those subjects in whom it was not observed.
Failure of Balloon Distension
Forty-one fractures were treated with BKP. The failure of the balloon distension occurred in 6 fractures (14%), so these fractures were treated with conventional vertebroplasty and the possible reasons associated with it have been discussed in the following paragraphs.
Fracture of Bone Filler
We treated 41 fractures with BKP. The fracture of the bone filler occurred only in 1 patient, (no. 13, M.S), further managed by wound dilatation. The sawing of the bone filler near the bone and the remaining lift inside the pedicle was treated by an administration of I.V third generation cephalosporin 1gm twice daily for 5 days. The cause of this problem was attributed to the late removal of the bonefiller till the P.M.M.A was hardened. The clinical results did not differ from those subjects in whom it was not observed.
DISCUSSION
Vertebral fractures are the most commonly reported fractures in patients who suffer from osteoporosis. The estimated prevalence of vertebral fractures in women aged between 50 years and older is between 25-26%. The lifetime risk for a 50-year-old woman to develop at least 1 traumatic vertebral fracture is 32%. Multiple vertebral fractures are found in about half of the reported cases such that 84% are associated with pain. While the incidence of vertebral fracture is high, a minority of vertebral fractures (about one-third) requires clinical diagnosis. In the U.S.A, osteoporotic vertebral fractures account for approximately 650,000 clinically detected fractures per year.9,10,11
Acute vertebral fractures may present with pain, although clinically silent fractures may account for one half of the radiographically visible vertebral fractures. Pain management under acute conditions is not standardized and inadequate data is available on the natural history of acute painful vertebral fracture. Common management strategies include rest, activity modification, and the use of local and/or systemic analgesics.9
Chronic pain, usually following multiple vertebral fractures, tends not to respond to the management strategies implemented for acute pain. The source of chronic pain after vertebral compression fracture is not thought to be the vertebra itself, but is believed to relate predominantly to a strain on muscles and ligaments secondary to kyphosis. This type of pain is not frequently improved with the use of analgesics and may be better addressed through performing exercises. Epidemiologic studies also suggest that vertebral compression fractures may be associated with long-term mortality. In a prospective cohort study of women older than 65 years, 10-year mortality was recorded to be proportional to the number of symptomatic vertebral compression fractures, rising from 19 per 1,000 person-years with no fractures to 44 per 1,000 person- years in women with 5 vertebral compression fractures.9,12
Pain can be caused by nociceptors in the bone itself, the disc complex, the perivertebral structures, through nerve compression, joint or muscle pain. Although most of the patients with this injury experience a benign and self-limited course of gradually resolving pain, a significant number continues to experience chronic pain, progressive kyphosis and disability. Long-term consequences include significantly decreased activities of daily living (ADL) accounting for five million limited activity days in the US.13
As a consequence, the patients suffer from an increased dependence on others, sleeping disorders and clinical anxiety, including reduced mobility. Finally, hyperkyphotic patients are at a risk of experiencing reduced pulmonary functions.14
As our population ages, the rate of osteoporotic fractures is expected to triple over the next few decades.15 An ideal strategy for the treatment of VCF should be towards relieving fracture related pain and improving the QoL. Kyphoplasty was designed to address the kyphotic deformity and to help realign the spine.16,17,18
The first reported case of kyphoplasty was published by Wong et al17 in the Journal of Women’s Imaging in 2000. They reported a study conducted on 85 patients. Over 90% of the patients indicated good or excellent pain relief. The height restoration was 62%. Lieberman et al19 presents a prospective clinical examination of phase-I investigation for the treatment of 30 patients with 70 Kyphoplasty procedures performed. Local cement leakage was observed in 8.6% of the reported cases. The restoration of the VB height was 47% in 70% of the cases, and in the remaining 30% of the cases, no measurable correction was achieved.
In 18 patients, 55 kyphoplasty procedures were performed. With respect to height loss, 34% was restored, and leakage was observed in 4% of the vertebrae.20 Theodorou et al21 reports the treatment of 15 patients with 24 BKP. Height restoration was best observed in the mid-vertebral body with 65% of the patients. Kyphosis correction was 9.5° in an average or was achieved by 62%. Pain was improved in all the patients after a follow-up of 6-8 months.21 Coumans et al22 presents a prospective study with kyphoplasty with a minimal follow-up of 1 year. Of 78 patients with 188 kyphoplasty procedures, 62% were available for a follow-up evaluation. They report five cases of asymptomatic extravasation SF36; Oswestry score and pain (VAS) did improve immediately post-operatively with a lasting effect at FU. The kyphosis correction is not mentioned.22
It has been reported that kyphoplasty can improve the kyphotic angle in the range of (3.4o to 8.8o) degrees. However, clinically, 34% of the cases of kyphoplasty do not show a demonstrable reduction in the kyphotic angle or restoration of the vertebral body height. One reason for inadequate height preservation in kyphoplasty is the loss of vertebral body height after balloon deflation, prior to the cement injection.23 Despite positioning the patient in a lordotic position (prone position with a concave curvature of the spine), compression forces of approximately 110 N are still exerted on the vertebrae and may contribute to the collapse of the created cavity.23,24 This is an inherent problem associated with the procedure of kyphoplasty.
In our study, we observed that the median kyphosis angle was 21.8° before the procedure and decreased by a median of 6.7° following the surgery. 60% of the patients experienced a reduction in kyphosis by more than 5° (Interquartile range of 10°). In an analysis of this subgroup of patients, faster pain relief was achieved compared with patients who showed minimal to no reduction in the kyphotic angle. The VCFs were then examined on the basis of the spinal region (thoracic or lumbar) in which it occured. 22 thoracic vertebral compression fractures were treated in 18 patients with a median improvement of 5.8° (range 0° to 17°) in sagittal alignment across the treated segments. Eighteen lumbar vertebral compression fractures were treated in 16 patients causing a 5.6° (range 0°-17°) median improvement in the angle of lordosis. If fractures that were considered reducible by BKP (defined as at least 5° improvement in sagittal alignment) were analyzed, 18 fractures in 18 patients showed an improvement in the sagittal alignment by 10° (range 6° to 17°). Statistical significance in the improvement of the sagittal angle from pre-operative to post-operative measurements reached the p<0.0001 level (Wilcoxon signed-rank test) for all the fractures combined, thoracic fractures, lumbar fractures, and reducible fractures.
Long-term follow-up will be required to determine whether the improvement in spinal kyphosis will reduce the disability, morbidity, or risks of developing subsequent VCFs associated with kyphotic deformity from osteoporotic vertebral fractures. The clinical and radiographic results showed that the effects of BKP are immediate and dramatic, and showed no evidence of deterioration with time. Alleviation of pain, reduction in the use of pain medications, and improved mobility occurred within the first few post-operative days to weeks and remained stable for the entire length of the study.
Pain relief after BKP was rapid and evident within the first week following the procedure. The pain relief was sustained for upto 6 months after the procedure. Most patients also reported a return to the pre-fracture functional levels. This effect was observed due to at least a 4-point decrease in the VAS pain score in 26 (87%) patients who achieved dramatic post-operative improvement whereas, in 2 cases, significant improvement was noted during the 3 months follow-up. On the other hand, 2 (7%) patients showed a further deterioration in the pain score. Twenty four (80%) patients showed constant improvement over a period of 6 months, suggesting further improvement in 7 patients following the 6 month period (23%).
There was a rapid and marked improvement in the QoL scores (ODI score) in this study. The mean pre-operative ODI score was 80, decreasing to 32 after 1 month postoperatively, and improving to 21 and 17 after the 3 month and the last follow-up, respectively.
The current study has several limitations. Firstly, the influence of the fracture etiology on the patient outcomes could not be assessed because of the relatively small number of subjects with secondary osteoporosis. Secondly, the current study, like most of the orthopedic literature on VCF treatment, lacks a control group. In 1999, one of the authors (S.R.G.) attempted to conduct a randomized controlled study comparing balloon kyphoplasty to non-operative care. Initiated at 39 US centers, this study was terminated after 2 years after having enrolled only 41 subjects. Although, the study was not extensive, a prominent improvement in pain and function after balloon kyphoplasty was evident relative to non-surgical care.25 This study was undertaken to document the long-term benefits of balloon kyphoplasty in a large cohort, as recently suggested.26
Davis et al27 found that in women, a prevalent vertebral fracture increases the risk of incidence of fractures anywhere in the spine by 30%. However, if the prevalence fracture was in L2-L5, the odd ratio increased from 1.3 to 5.7 compared to women with no prevalent fracture. Their results suggested that the increased risk of fracture in women with prevalent fractures extends beyond the nearby vertebrae, which can affect the vertebrae both above and below the prevalent fracture. Existing fractures are strong and independent predictors of the risk of future vertebral fractures.27 Thus, the vertebra adjacent to the site of cement injection is at no higher risk of fracture than any other vertebrae. The authors discuss three reasons as to why a prevalent fracture increases the risk of incident fracture.
First, the prevalent fracture is an indicator of impaired bone strength. Second, the loading is altered and third, the prevalent fractures are also due to other risk factors for developing fractures, such as falling.
Jenson and Dion28 studied 109 patients with 174 fractures retrospectively and found no statistical difference in the rate of adjacent level fractures between patients who returned with new fractures following vertebroplasty and a control group consisting of patients who presented with multiple fractures of the same acuteness.
Additionally, some investigators have suggested that vertebral body augmentation may alter stiffness, leading to an increased risk of subsequent adjacent-level VCFs. However, the biomechanical basis for this theory is poorly documented.29,30,31
As our patient population was so small and the duration period of follow-up was relatively short, we were not able to conclude anything definite about the problem of adjacent-level fractures.
In our study, evaluation of intra-operative and post-operative radiographs revealed extra vertebral cement leaks in 11 (27%) of the 41 vertebral fractures treated. From our experience, it seems that the use of BKP limits the occurrence of extravasation supported by our results indicating reduced extravasation than that reported by conventional vertebroplasty.
Many theories about extravasation during the osteoporotic vertebral fractures augmentation continue to exist. During vertebroplasty and kyphoplasty, a bone filler is introduced into the fractured vertebral body to stabilize the fracture. For vertebral augmentation procedures, PMMA has been the most widely used bone filler, and most of the serious complications reported are related to the leakage of PMMA outside the confines of the vertebral body during injection.32
The PMMA may exit the vertebral body through deficiencies or fractures in the vertebral cortex or by injection of the cement into the vertebral venous system.26 Leakage of the cement through the vertebral cortex may result in direct injury to or compression of the adjacent structures such as the spinal cord.
When injected cement enters the venous system, it has the potential to fill the epidural veins and cause spinal cord or nerve root compression, or PMMA pulmonary embolism.33
In addition, high-pressure interosseous injection of cement may lead to embolization of methylmethacrylate monomer and bone marrow contents of the lungs, with negative cardiopulmonary sequelae.29,34,35,36,37 Pressurization of PMMA during joint arthroplasty surgery has been associated with occasional cardiovascular collapse. During vertebroplasty, the high-pressure injection of low-viscosity cement directly into cancellous bone makes it difficult to control the cement flow in the vertebral body, thus creating an unpredictable risk of cement extravasation outside the vertebral body. In fact, extra vertebral cement extravasation commonly is seen during vertebroplasty. However, proponents of this technique have reported infrequent clinical sequelae of the leakage.
Kyphoplasty is an attempt to address the shortcomings of vertebroplasty (i.e., the inability to correct spinal deformity and high- pressure injection of cement into the fractured vertebral body). During kyphoplasty, the creation of an intervertebral cavity with the increasing block tariffs (IBTs) allows for a range of filler options, placing it under lower pressure.
In addition, the IBT compacts the trabecular bone, which may seal potential osseous or venous leak paths. When PMMA is used, it can be placed in the partially cured (viscous) form into the created cavity, which may reduce the risk of leakage.
Cadaver and clinical studies support the evidences of reduced leakage rate with kyphoplasty. Belkoff et al38 reported extravertebral PMMA (Simplex P) leakage in 5 of the 8 fractured human cadaveric vertebral bodies treated with vertebroplasty, with no cement leakage seen in the fractured vertebra treated with kyphoplasty.
In a similar cadaver model, Belkoff et al38 reported that when vertebroplasty was performed with the cement of varying materials, cement leaks occurred in 8 of the 12 treated vertebral bodies.
In a clinical Phase 1 efficacy study, Lieberman et al19 reported 30 patients who had osteopenic VCFs treated with kyphoplasty at 70 vertebral levels and noted extravertebral cement leakage at six levels (8.6%). No neurological complications occurred.
In a report of the initial 1471 VCFs treated with kyphoplasty in the United States, only one case of neurologic compromise related to cement intrusion into the spinal canal or neural foramen was mentioned.16 With the vertebroplasty contrast injection, cement leakage was observed through the vertebral cortex in 60% of the injections, raising a concern for direct PMMA leakage outside the bony spine.
CONCLUSION
In this study, all PMMA extravasations were asymptomatic; the cement remained in the immediate vicinity of the treated vertebrae. Forty one fractures were treated with BKP. The failure of balloon distension occurred in 6 fractures (14%) which were managed with conventional vertebroplasty and the exact cause behind that is still unclear. But, we can conclude from our experience, the possible causes behind a similar situation. Firstly, when the balloons were inserted in long standing old fractures (more than 2 months), tight ligaments and fibrosed disc spaces prevent the inflation of the balloon. Secondly, when the balloons were not inserted into the fractured area. Finally, when the balloons ruptured due to bone spikes.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.





















