INTRODUCTION
The acute increase in type 2 diabetes mellitus (T2DM) is one of the most serious medical problems today. T2DM patients were about 385 million in 2014 and increase to 592 million by 2035.1,2 Furthermore, the global health expenditure for diabetes has been approximately 11% of total medical spending on adults in the world.1,2 T2DM is characteristic for the deterioration of pancreatic β-cell function and insulin resistance in the liver and peripheral tissues. A combination of these factors, T2DM has an overproduction of hepatic blood glucose, elevation of fasting blood glucose, and insufficient glucose uptake by muscle cells which would increase postprandial blood glucose.3 Furthermore, several pathophysiological impairments have played a role in the progression and development of T2DM, such as liver, muscle, dysfunction of pancreatic α-cells with elevated glucagon value, fat cells with accelerated lipolysis and kidney with increased glucose reabsorption.1,2
T2DM has a chronic elevation of blood glucose levels, resulting in microvascular complications with neuropathy, retinopathy, nephropathy, and macrovascular complications with ischemic heart disease, cerebrovascular disease, peripheral arterial disease.4 Therefore, clinical research and therapy have been continued for the achievement of optimal glycemic control associated with optimal management of body weight, blood pressure, and lipid levels for years. Recently, several new anti-diabetic medicines have been developed. They have some beneficial effects for lowering HbA1c, body weight, hypoglycemia incidence and others.5
As to the history of endocrinology and diabetes, Elrick and colleagues revealed interesting findings. It was reported that, when an oral glucose tolerance test was conducted on a healthy person, markedly increased insulin secretion was observed compared to an intravenous load test.6 From this report, it suggested the presence of incretin (intestine secretion insulin (INCRETIN)) as a substance that enhances insulin secretion in the intestinal mucosa.
Later gastric inhibitory polypeptide (GIP) was identified by purification of intestinal hormone and glucagon-like peptide-1 (GLP-1) showing incretin action was reported.7 Successive studies revealed the role and mechanism of GLP-1.8
The function of GIP-1 has been reported to have several functions as follows: stimulating insulin secretion, inhibiting glucagon secretion, promoting glucose production and glucose uptake in the liver, increasing glucose uptake and glycogen production in skeletal muscle, decreasing food intake for the central nerve and delay of gastric emptying and so on.
Recently, an agent was developed for T2DM known as Glucagon-like peptide-1 receptor agonists (GLP-1 RAs). They have a remarkable efficacy and safety profile distinct from other anti-diabetic agents.9 GLP-1 RAs could decrease blood glucose level by several function or mechanism, which are an enhancement of glucose-induced insulin secretion, suppression of glucagon secretion and delayed gastric emptying.10,11
As described above, in recent years, GLP-1 RAs and DPP-4 inhibitors for diabetes have been widely used. Under these circumstances, the authors have been treating patients with lifestyle-related diseases including diabetes and obesity for many years.12-14 In particular, we have been conducting clinical research on low carbohydrate diet for numerous cases, research on metabolism related to ketone bodies and carbohydrates, treatment of diabetes, etc., reporting on glucose limitation, blood glucose variation, ketone body and carbohydrate.14-16
We recently administered dulaglutide that is one of GLP1 RA for two elderly patients with T2DM, which showed interesting clinical course. Then we have described these cases and discussed in detail.
CASE REPORTS
Case 1
An 88-year-old male patient who has been formerly diagnosed with type 2 diabetes mellitus (T2DM) at the age of 73. Later he had been given oral medicine for years, and recently he got treated with insulin administration twice by a mixed type of 3:7 in a different clinic. From 2014, he started getting treated at our diabetes department of our hospital.
On physical examination, he showed 163 cm in height, 67 kg in weight, BMI 25.2 at first visit, and his vitals and consciousness are normal, and lung, heart, abdomen and neurological findings were unremarkable. Laboratory data were as follows: postprandial glucose-120 min 156 mg/dL, HbA1c was 7.4%, Hb 11.4 g/dL, GOT 22 IU/mL, GPT 26 IU/mL, r-GTP 26 IU/mL, BUN 24 mg/dL, Cre 1.1 mg/dL, TG 334 mg/dL, LDL 117 mg/ dL.
The patient had some other medical problems, such as hypertension, arteriosclerosis, benign prostate hypertrophy (BPH) and mild cognitive impairment (MCI) without difficulties of Quality of Life (QOL)/Activities in Daily Living (ADL) in daily life. He has been given several oral medicines including aspirin 100 mg, valsartan 20 mg and tamsulosin hydrochloride 0.2 mg. For the fine adjustment of treatment for T2DM, glimepiride dosage from 0.5-3 mg per day has been added according to the value of HbA1c and daily profile of blood glucose.
Progress course of case 1 is shown in Figure 1. Until May 2016, his control status of blood glucose was good and stable on the administration of liraglutide 0.9 mg/day. C-peptide indexes (CPI) were 2.5 and 3.3 which were satisfactory. Then, GLP-1 analogue was switched from liraglutide to dulaglutide. The reason was that the injection can be lessened from once per day to once per week by the strong hope of the patient and family.
Figure 1. Clinical Course in Case 1
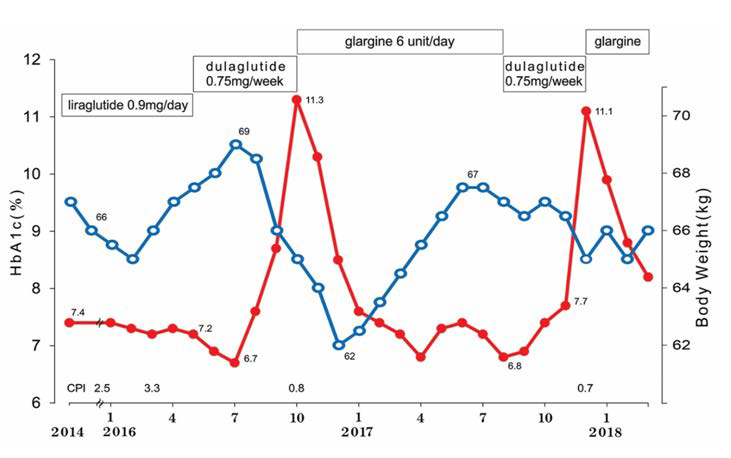
After the switch of the GLP-1 agent, the clinical course was stable for 3-4 months. However, a daily profile of blood glucose and HbA1c was gradually elevated. The patient was admitted for hyperglycemia with blood glucose 394 mg/dL and HbA1c 11.3% in October, 2016. After that, long-acting insulin glargine was applied for the treatment. HbA1c was good and stable for 8 months until August, 2017. The patient and the family hoped again to change from glargine to dulaglutide for their inconvenience and forgotten the episode of injection every day.
There was good glucose control for 3 months, but blood glucose and HbA1c was again elevated in December, 2017 with the value of 518 mg/dL and 11.1%, respectively. He was admitted and treated with enhanced insulin therapy by insulin glargine and humulin R, which are long-acting and short-acting insulin. After his improvement and discharge from the hospital, he has been on glargine 12-14 units/day. After the switch of the GLP-1 agent, the clinical course was stable for 3-4 months. However, a daily profile of blood glucose and HbA1c was gradually elevated. The patient was admitted for hyperglycemia with blood glucose. As mentioned above, case 1 has been treated as basal supported oral therapy (BOT), which were glargine (insulin) and glimepiride (sulphonylurea agent). The combination of liraglutide and sulphonylurea agent indicated satisfactory result for long months, but dulaglutide and sulphonylurea agent had an effect of only about 3 months.
Case 2
An 83-year-old male patient who has been treated as T2DM at another clinic for more than 20 years. He has been in the nursing home in recent few years. He was admitted to our hospital in October, 2016 for further evaluation and treatment.
On physical examination, he showed 152 cm in height, 40.3 kg in weight, BMI 17.4 at first visit, and his vitals and consciousness are normal, and lung, heart, abdomen, and neurological findings were unremarkable. Laboratory data were as follows: postprandial glucose-120 min 207 mg/dL, HbA1c was 8.5%, Cpeptide- immunoreactivity 5.2 ng/mL, CPI=1.35. Hb 11.0 g/dL, WBC 5200/μL, Plt 17.4×104 /μL, GOT 14 IU/mL, GPT 11 IU/ mL, r-GTP 11 IU/mL, LDH 163 IU/mL, BUN 29 mg/dL, Cre 1.6 mg/dL, uric acid 3.7 mg/dL, Na 143 mEq/L, Cl 106 mEq/L, K 4.0 mEq/L, TG 83 mg/dL, HDL 31 mg/dL.
The patient has other medical problems, such as hypertension, chronic heart failure, chronic renal failure and mild cognitive impairment (MCI). Consequently, the list of oral medications per day were as follows: 1) linagliptin 5 mg qd., voglibose 0.3 mg tid., repaglinide 0.5 mg tid. for diabetes, 2) azosemide 60 mg qd., telmisartan 40 mg qd., amlodipine besilate 5 mg qd. and carvedilol 5 mg qd. for hypertension and chronic heart failure, 3) febuxostat 10 mg qd., calcium polystyrene sulfonate 50 g bid., calcitriol 0.5 µg qd., and epoetin beta pegol 50 μg per month for chronic renal failure, 4) esomeprazole magnesium hydrate 20 mg qd., donepezil hydrochloride 5 mg qd and clopidogrel sulfate 50 mg qd. for Gastroesophageal Reflux Disease (GERD), dementia and anticoagulation therapy. Progress course of case 2 is shown in Figure 2. Until May 2017, his glycemic control had been good and stable on the administration of degludec 3-5 unit/day. C-peptide index (CPI) was 1.35 which seemed to be stable. HbA1c value increased to 8.0% in June 2017, and then dulaglutide was started for the expectation for improving glycemic variability and for convenient therapeutic injecting of GLP-1 analogue once per week by the hope of the patient and family.
Figure 2. Clinical Course in Case 2
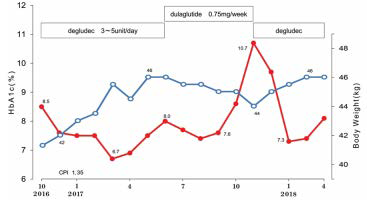
After starting dulaglutide in June 2017, the clinical course was stable and HbA1c value was decreased for 3 months. However, in October, blood glucose and HbA1c were increased up to for 3-4 months. However, daily profile and blood glucose and HbA1c were gradually increased. On November, postprandial glucose-120 min and HbA1c were 592 mg/dL, and 11.2%. He was immediately admitted to the hospital on urgent basis.
GLP-1 agent was discontinued and degludec and humulin R was administered in order to maintain stable glucose variability. With this treatment, HbA1c was gradually decreased and it was 7.3 % in January 2018. Regarding the changes in body weight (Figure 2), it increased for a few months during twice treatment of degludec, and it decreased for a few months during treatment of dulaglutide.
As mentioned above, case 2 had been treated as basal supported oral therapy (BOT), which were degludec (insulin) and glimepiride (sulphonylurea agent). Combination therapy of degludec and sulphonylurea agent revealed stable condition until June, 2017. After that dulaglutide was first tried and seemed to show satisfactory effect. However, the efficacy has lasted only about 3 months, associated with the exacerbation of blood glucose profile and HbA1c level.
Basal Information of GLP-1 and Dulaglutide
GLP-1 relies on insulin secretion and stimulates glucose. Several types of GLP-1 RA have been developed and have been clinically used. It is thought that GLP-1 RAs administered alone does not have a risk of hypoglycemia.17 GLP-1 RAs also promote weight reduction, probably through the suppression of appetite by the activation of GLP-1 receptors in the brain.18,19 They can decrease blood pressure by increasing urinary sodium excretion with vasodilation, resulting in lowering blood pressure. GLP-1 RAs can improve postprandial hyperlipidemia by suppressing the production of apolipoprotein B-48 and triglyceride absorption.10,11 Furthermore, they can suppress inflammation responses by reducing production of inflammatory cytokines and macrophage infiltration.10,11 Therefore, GLP-1 RAs have indicated beneficial effects for decreasing blood glucose and weight reduction, blood pressure, lipid levels and inflammatory responses.
Dulaglutide is a novel, long-acting GLP-1 RA which was developed as a once-weekly subcutaneous injection for the treatment of T2DM (Eli Lilly and Company, USA).20 It consists of two GLP-1 analogs covalently linked by a small peptide to a human immunoglobulin G4 (IgG4-Fc) region-specific heavy chain. The GLP-1 moieties contain amino acid substitutions protecting from inactivation by dipeptidyl peptidase-IV (DPP-4), while the linker peptide is maintaining the potency of the GLP-1 analog. The human IgG4-Fc is modified by substituting several amino acids to reduce interaction with high-affinity Fc receptors, cytotoxicity, and immunogenicity.21 The half-life is approximately 5 days, and time to peak concentration after a single 0.75 mg dose is approximately 3 days.
Dulaglutide is a once a week formulation. The beneficial points are high blood glucose improving the effect, easy injection procedure, and low frequency of antibody appearance. It is also effective for patients who have poor compliance or cognitive impairment and require nursing care and others. Concerning clinica efficacy, it showed a dose-dependent reduction in HbA1c and acceptable safety profile in the randomized, double-blind, placebocontrolled, parallel-group, 12-week study.22
Concerning dulaglutide, decrease in absorption rate and clearance after injection is found due to increase in molecular weight. Therefore, the half-life of blood concentration is extended to 4-5 days, and stable blood concentration can be maintained for 1 week.23
Dulaglutide is manufactured by Eli Lilly, where the trade name is Trulicity, and it has been approved by The European Medicines Agency (EMA) and the United States Food and Drug Administration (FDA).20.23 There are reports for the efficacy of dulaglutide. Several studies have been reported as the Assessment of Weekly Administration of dulaglutide in Diabetes (AWARD) program.20.23 As a study of dose setting, two-years of study was conducted with AWARD-5.24 The standard dose has become 1.5 mg/week in the United States and Europe. Meanwhile, 0.75 mg/week has been the standard usage method in Japan. HbA1c change was-1.60% at 26 weeks in 855 T2DM patients.25There are 4 subgroups according to age (<65, >65) and BMI (<25, >65), in which elder/light and elder/heavy subgroups revealed -1.68% and -1.72%, respectively.26
One year clinical research was carried out, resulting from the improvement of HbA1c was found at 14-52 weeks with -1.65%±0.13%.27 Furthermore, there was randomized, open-label, parallel-group, 26-week study with 361 patients with T2DM receiving sulphonylureas and/or biguanides, in which dulaglutide or glargine were added and compared in 2 groups. The decrease in HbA1c after 26 weeks was -1.44% vs. -0.90%, respectively, with a significant difference.28
Effects of once-weekly dulaglutide on kidney function in patients with T2DM was investigated in phase II and III clinical trials.29 Integrated data from 2005 T2DM patients were used to evaluate the influences on estimated glomerular filtration rate (eGFR), urine albumin-to-creatinine ratio (UACR) and kidney adverse events (AEs). The results were dulaglutide treatment for T2DM did not affect eGFR and slightly decreased albuminuria.
DISCUSSION
In this report, two cases seemed to show an attenuated effect of GLP-1 RAs in a few months. This may be related to the function of GLP-1 RAs including short-acting and long-acting types.
In general, GLP-1 RAs are categorized into 2 groups, which are short- and long-acting agents according to their halflives. They would change the mechanism of function for glucose lowering effects.30,31 The former seems to act mainly on gastric motility and lowers blood glucose after meals. On the other hand, the latter mainly acts on the pancreas to regulate insulin and glucagon secretion, thereby leading to stabilization of blood sugar. In addition, it is pointed out that the high-molecular-weight formulations found in some long-acting forms may attenuate the appetite suppressing effect due to the passage of brain blood barrier.32,33
Actually, several studies of GLP-1 RAs have been reported. In previous studies, long-acting liraglutide was found to be rather superior to the short-acting exenatide, comparing the achievement of composite end-points.34
Recently, there are lots of studies reported on short-acting (exenatide, lixisenatide) and long-acting GLP-1RAs (liraglutide, exenatide long-acting release, albiglutide, dulaglutide and semaglutide).35-38 These agents are available in the clinical settings, allowing comparison of the study of composite end-points in future. Moreover, long-acting GLP-1 RAs seem to have weight reduction effects according to their molecular weight and permeability across the blood-brain barrier, possibly by affecting their accessibility to GLP-1 receptors in the brain.39,40 Through various cases treated with GLP-1 RAs, further investigation would be expected concerning pathophysiological comparison and difference between long-acting and short-acting agents.
As to dulaglutide study, 492 T2DM cases were studied for 26 weeks in 3 groups including dulaglutide 0.75 mg/week, liraglutide 0.9 mg/day and placebo.41 Changes in HbA1c were -1.43%, -1.33%, and -0.14%, respectively, in which the former two were equivalent.41
Fasting blood glucose levels were -39.2 mg/dL, -39.8 mg/dL, +1.0 mg/dL, respectively, with equivalent efficacy. Interestingly, the group of dulaglutide 0.75 showed a decrease in urinary albumin excretion rate and triglyceride value compared to placebo. Regarding the adverse effects, several symptoms were observed such as nasopharyngitis, gastrointestinal symptoms, constipation, diarrhoea, nausea and decreased appetite. As pancreatic enzymes, amylase, and lipase were slightly elevated, and the increase of lipase was 18.5%, 29.8%, 4.9% in the three groups, respectively. Antibody at dulaglutide 0.75 appeared at only 1.1%, but no neutralizing antibody appeared.41
Subsequently, in the 52-week study, the two groups of dulaglutide 0.75 mg/week and liraglutide 0.9 mg/day showed -1.39% and -1.19%, respectively, with slight superiority in the former.42 In western countries, these two agents have been used twice daily.. According to the results of AWARD-6, the two groups of dulaglutide 1.5 mg/week and liraglutide 1.8 mg/day at 26 weeks showed the changes in HbA1c, which are equivalent to -1.42% and -1.36%, respectively.40
In AWARD-9 study, the addition of weekly dulaglutide vs. the addition of placebo to titrated glargine was compared.43 Subjects were 300 T2DM and phase III, double-blind, parallelarm, placebo-controlled study were randomized to weekly subcutaneous injections of dulaglutide 1.5 mg or placebo with titrated daily glargine. The results were that decrease HbA1c at 28 weeks was -1.44% with dulaglutide/glargine and -0.67% with placebo/ glargine with significant difference (p<0.01). The hypoglycemia’s rate was 7.69 and 8.56, respectively without any difference. These findings suggest that weekly dulaglutide 1.5 mg added to basal insulin would be an efficacious and well-tolerated treatment option for patients with T2DM.43
As mentioned above from the study on dulaglutide once a week by injection, blood glucose and HbA1c for T2DM are decreased with enough efficacy. Furthermore, adverse effects are not clinically significant. Judging from the clinical outcome to the third phase internationally, dulaglutide seems to be effective and safe GLP-1 RA. It will be useful for diabetic patients of all pathological conditions pharmacologically. Especially, it can be applicable for young and elderly patients with some impairment and dementia with some impairment and dementia with insufficient QOL/ADL ability.
As current 2 cases, recent treatment of type 2 diabetes has moved from the previous Basal Supported Oral Therapy (BOT) to a new Basal supported post- Prandial GLP-1 therapy (BPT). The former is basal insulin + oral medicine, and the latter is basal insulin + GLP-1 RA treatment.
There is a comparison study between BOT and BPT. For the comparison group, 557 T2DM patients were classified into 2 groups.44 Degludec/liraglutide group and glargine group showed that basal HbA1c as 8.4% vs. 8.2%, the changed HbA1c at 26 weeks as -1.81% vs. -1.13% respectively with meeting criteria for non-inferiority (p<0.001) and confirmed hypoglycemic episodes as 2.23 vs. 5.05 per year with estimated rate ratio as 0.43 (p<0.001).
Consequently, BPT may have a superior effect than BOT, and benefits of BPT include stabilization of blood glucose fluctuation and good health-related quality of life (HRQOL).
HRQOL and cost-effectiveness have been also important aspects in addition to management of glycemic control for prevention of diabetic complications. GLP-1 RAs are treated by injections, which sometimes decrease HRQOL for the patients. Moreover, considerably higher medical costs are needed compared with that of oral anti-diabetic drugs. There are few reports concerning HRQOL and medical costs regarding GLP-1 RAs so far, expecting the future development of clinical administration study.35,36 Expecting the future development of clinical administration studies.
Liraglutide therapy is necessary to receive a once-daily injection. In contrast, other long-acting GLP-1 RAs are given by injection only once weekly. This merit may bring better adherence and prevent the deterioration of HRQOL.
Combined therapy of basal insulin and GLP-1 RAs which is called BPT, has been recently gaining attention due to their beneficial and complementary action.45,46 When GLP-1 RAs are added to insulin treatment, the effects would include weight reduction without increasing hypoglycemic risk, in comparison with the insulin treatment with increased dosage. In the recent metaanalysis, BPT decreased HbA1c value which is similar to basalbolus insulin therapies.46 Furthermore, BPT can improve HRQOL by reducing injection frequency. For T2DM patient with severely reduced β-cell function, the combined therapy can give beneficial effects and achieve optimal glycemic control by GLP-1 RAs.45,46 In order to have optimal glycemic control, the extent remaining β-cell function would be speculated according to the actual dosage units of insulin and GLP-1 RAs.
The long-acting type slowly acts on pancreatic β-cells, improves insulin secretion ability, and tends to improve blood glucose control. In fact, any GLP-1 RA can be selected on a caseby-case basis, including differences in devices or syringes.
In summary, we reported that 2 T2DM cases showed an attenuated effect of dulaglutide in a few months. For this clinical situation, several possibilities might be speculated as follows:
1) Antibody production could be considered. The frequency of appearance of dulaglutide anti-drug antibodies was very rare, where only 1 out of 136 cases28 and 3 out of 281 cases.41Then, it is not likely due to antibody production in 2 cases.
2) GLP-1 RAs were made from human-origin and lizard-origin (exendin-4). The former includes that 1 mal/day in liraglutide and 1 mal/week in dulaglutide, and the latter includes that 1 mal/ day in exenatide (byetta)/lixisenatide (liximia), and exenatide (videlion) in 1 mal/week. Since dulaglutide is human-origin, it does not probably influence the formation of an antibody. Moreover, there has not been clarified whether these two agents have any difference of function.
3) The changes in body weights of 2 cases were found, where they increased during treatment of liraglutide, glargine and degludec, and they decreased during treatment of dulaglutide. The latter would be one of the merits of dulaglutide that has been reported so far.18,19,39,40,45,46 However, it is not known whether it has any relations with clinical efficacy or any other adverse effects. This point would be further evaluated through clinical studies.
4) Current two cases have seemed to have similar pathophysiological problems, such as late elderly person, a little impaired cognitive function and declined renal function. In the clinical course, the have not suffered from any changes of nutritional or hepatic function, or inflammatory status during the period. Any remarkable changes have not found which were related to subacute exacerbation of glycemic control.
5) There is a possibility of the subacute decreased ability of insulin secretion in the β-cell. We had measured C-peptide index (CPI) a few times which may give a key to the changed pathophysiological situation. In the cases, we would consider all possible related factors influencing to insulin secretion, administration of insulin and GLP-1 RAs and others.
CONCLUSION
This report described 2 cases of T2DM which showed interesting clinical course. The effect of dulaglutide that is one of the GLP-1 RAs was attenuated in a few months. We speculated the possibilities related to the function and comparison of GLP-1 RAs. GLP-1 RAs treatment is increasing widely in the clinical practice, expecting that this report would be useful resource for management of the T2DM.
ETHICAL CONSIDERATIONS
The current study was conducted in compliance with the ethical principles of the Declaration of Helsinki. Furthermore, it was also with Japan’s Act on the Protection of Personal Information along with the Ministerial Ordinance on Good Clinical Practice (GCP) for Drug (Ordinance of Ministry of Health and Welfare No. 28 of March 27, 1997).
As regard to this study, we have established an ethical committee in the Yoshinogawa Hospital that included the president, the vice-president, the head-nurse of the nursing department, director of the Pharmaceutical department, director of the administration and expert in the medical/legal specialty. We have discussed and made confirmation that this study is valid and agreed with all members without any problems. Furthermore, informed consents and written paper agreements have been obtained from both the subjects.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.







