INTRODUCTION
Avocado (Persea Americana, Lauraceae) is an important tropical crop that is rich in unsaturated fatty acids, fiber, vitamins B and E, and other nutrients. The Hass avocado is the most important variety grown commercially. The seed of the Hass avocado accounts for approximately 16-20 % of the total weight of the avocado fruit and is considered a low-value waste product. In 2017, the total avocado shipped into the USA was 994,340 metric tons, including domestic production of 91,660 metric tons.1 Most of this imported from Mexico, the world’s largest grower of avocado seed. The amount of seed generated in the USA in 2016 was 159,100 metric tons.1
Ethno-pharmacological studies of the Aztec and Maya cultures have reported the use of decoctions of avocado seeds for the treatment of mycotic and parasitic infections, diabetes, inflammation, and gastrointestinal irregularity. Our previous review highlights multiple potential applications of avocado seeds including anti-inflammatory effects.2
Avocado seeds are rich in polyphenols and contain a large number of different classes of phytochemicals. The seed has higher polyphenol content and greater antioxidant activity than the pulp.3,4,5 Wang et al (2010) have reported the presence of catechin, epicatechin, and A- and B-type procyanidin (PAC) dimers – hexamers in the seed. The seeds have also been reported to contain phytosterols, triterpenes, fatty acids, furanoic acids, and abscisic acid.5 Among the compounds identified in CASE are perseitol, abscisic acid, epicatechin/catechin, PAC B2 and salidroside.6 Melgar et al. (2018) identified many polyphenolics from the hydroethanolic extract of seed, including isorhamnetin-glucuronide, catechin, epicatechin, trans-3-O-Caffeoylquinic acid, B-type PAC dimer and trimer, cis 3-O-caffeoylquinic acid, and cis-3-p-coumarouylquinic acid. These authors compared the polyphenolic content of seeds and peels and found the peels to have 3-fold higher polyphenolic content but only around twice the antioxidant activity. The extracts also displayed bactericidal and fungicidal characteristics.7
We have previously reported that the avocado seed when crushed, a stable orange color develops and we have investigated the potential use of colored avocado seed extract (CASE) as a food additive.8 The principal colored compound in CASE has been identified as a novel glycosylated benzotropolone-containing polyphenol.9 A large number of studies have demonstrated the potential anti-inflammatory activities of benzotropolone-containing natural products such as theaflavins from black tea. Based on these previous studies on the anti-inflammatory activity of benzotropolones, the potential usefulness of a CASE as a food additive, and the general lack of studies on the anti-inflammatory activity of avocado seed extracts, an investigation of the potential anti-inflammatory activity of this new extract was warranted. We hypothesized that CASE would exhibit dose-dependent inflammation inhibitory activity in vitro. In the present study, we examined the effect of the colored avocado seed extract (CASE) on LPS-induced inflammatory responses of RAW264.7 murine macrophages.
MATERIAL AND METHODS
Reagents
Ripened avocado (Persea Americana, Hass variety) were sourced locally and stored at 4 o C until use. The ELISA for PGE2 was obtained from Cayman (Ann Arbor, MI, USA). ELISAs IL-6, Il1β, TNF-α ELISA kits were obtained from R&D systems (Minneapolis, MN, USA). Lipopolysaccharide and Greiss reagent was obtained from Sigma (St. Louis, MO, USA). The phospholipase A2 enzyme assay was purchased from Invitrogen (Carlsbad, CA). Antibodies against JNK, MAPK, phospho MAPK, NF-kB, iNOS, COX2, and β actin were purchased from Cell Signaling (Danvers, MA). All other reagents were of the highest grade commercially available.
Preparation of CASE
CASE was prepared as previously described8. In brief, avocado seeds were separated from the fruit, washed and peeled. Seeds were ground in 0.7 volume of deionized (DI) water using a Waring Blender. The resulting paste (pH 6.4) was incubated at 24o C for 35-min. The colored paste was transferred to a beaker, an equal volume of methanol added, and the mixture sonicated for 20 min, an additional 2 volume of methanol was added, and the mixture centrifuged at 1200 × g for 10 min. Methanol was removed using a rotary evaporator and the water removed by freeze-drying. Stock solutions (200 mg/mL) were prepared in dimethyl sulfoxide and stored at -80o C.
Cell Culture and Viability
RAW264.7 cells were maintained in log phase growth with Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/mL penicillin and 100 μg/mL streptomycin at 37o C under a humidified CO2 : air (5:95) atmosphere.
The effect of CASE on cell viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. In brief, cells were seeded (104 cells/well) in 96 well plates and allowed to attach overnight. The cells were co-treated with 1 µg/mL LPS and CASE for 24 h. After CASE treatment, cells were combined with MTT and absorbance read at 540 nm. The viability of treated cells was normalized to LPSonly treated controls.
Modulation of Inflammatory Cytokines
RAW264.7 cells were seeded (104 cells/well) in 96 well plates and allowed to attach overnight. Cells were then co-treated with CASE and 1 µg/mL LPS for 24 h in serum complete medium IL-6, IL-1β and TNF-α levels in the medium were determined by ELISA as per manufacturer’s instructions. The levels of these cytokines were compared to unstimulated cells and LPS stimulated control cells.
NO Production
RAW264.7 cells were plated and stimulated as above. The quantity of nitrite in the culture medium of CASE-treated cells was measured after 24 h as an indicator of NO production using Griess reagent. Briefly, 50 µl of cell culture medium was mixed with 50 µl of Griess reagent, the mixture was incubated at room temperature for 20 min and the absorbance at 540 nm was measured. The values were expressed as a percentage of LPS-only treated cells. Prostaglandin E2 Production PGE2 levels in media of CASE/LPS co-treated and LPS-stimulated RAW264.7 cells were measured using a PGE2 EIA monoclonal ELISA kit (Cayman Co., Ann Arbor, MI, USA).
Phospholipase A2 Inhibition Assay
Inhibition of PLA2 was examined using a commercially available fluorometric enzyme method. Buffered PLA2 solution (4 U/ well, pH 8.9) and CASE were combined in a 96-well plate. A fluorogenic PLA2 substrate (Red/Green BODIPY PC-A2, 1.5 μM) was dispensed to each well to start the reaction. After incubation at room temperature in the dark for 10 min, fluorescence was determined at λex=485 nm and λem=538 nm (Fluoroskan Ascent FL, Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA). Kinetic analysis of inhibition by PLA2 was carried out similarly but with the following modification–the concentration of CASE was held constant while the substrate concentration (0.5-4 μM) was varied.
Western Blot
Preparation of whole cell lysate: RAW264.7 cells (106 ) were seeded in 75 cm2 flasks for 36 h. The media was replaced with media containing 1 µg/mL LPS and CASE at 5 or 6 µg/mL and incubated for 24 h. The cells were washed with PBS, scraped off and centrifuged at 1200 × g. The cells pellet was combined with lysis buffer(25 mM 3-(N-morpholino) propanesulfonic acid, 2 mM Ethylenediaminetetraacetic acid (EDTA), 10% glycerol, 0.5% Nonidet P-40 and 0.02% sodium azide) containing 1:100 phosphatase inhibitor I, phosphatase inhibitor II and a protease inhibitor. The samples were mixed and disrupted by freezethawing
Preparation of nuclear lysate: The cells were treated as above and scraped and centrifuged at 800 × g for 10 min at 4 o C. The cell pellet was suspended in buffer A (10 mM 4-(2-hydroxyethyl)- 1-piperazineethanesulfonic acid (HEPES), pH 7.9, 1.5 mM MgCl2 , 10 mM KCl, 0.5 mM dithiothreitol (DTT), 0.1% Nonidet P40), incubated on ice for 10-min and centrifuged at 12,000 × g for 2-min at 4 o C. The pellet was re-suspended in buffer B (10mM HEPES, pH 7.9, 1.5 mM MgCl2 , 0.4 M NaCl, 0.5 mM DTT. 0.2 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride (PMSF) and 25% glycerol). The tubes were then vortexed and incubated on ice for 15-min with mixing every 5-min. They were then centrifuged at 10,000 × g for 10-min at 4 o C. The supernatant was removed and used as nuclear fraction.
Immuno blots: Protein (60 μg total or 20 μg for nuclear extract) was resolved by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes. After blocking for 1 h with blocking buffer (Li-Cor, Lincoln, NE), the membrane was incubated with respective primary antibody overnight. The bands were visualized using a fluorescent-conjugated secondary antibody using a Li-cor Odyssey Infrared system (Lincoln, NE).
Data Analysis
For kinetic analysis of PLA2, Michaelis Menten plots were generated using Graph Pad Prism (San Diego, CA, USA), and the maximum velocity (V-max), Michaelis Menten constant (Km), and mode of inhibition were determined from those plots. All other data were analyzed by one-way analysis of variance (ANOVA) with Dunnett’s post-test. p-values < 0.05 were considered as statistically significant. Data are presented as the mean±SD unless otherwise specified.
RESULTS
Cytotoxicity
The viability of RAW264.7 cells was determined after co-incubation with LPS and CASE for 24 h. At concentrations of CASE less than or equal to 6 µg/mL, cell viability was greater than 80% (data are not shown). Hence, only concentrations up to 6 µg/mL were used for further experiments.
Inhibition of Pro-Inflammatory Cytokine and Nitric Oxide Production by CASE
The levels of IL-6, IL-1β and TNF-α were measured in the media of LPS-stimulated RAW264.7 macrophages after co-treatment with CASE for 24 h. A dose-dependent reduction in the concentration of IL-6 was observed with a significant reduction at concentrations greater than 5μg/mL(Figure 1). The levels of IL-1β and TNF-α were significantly reduced by treatment with all the concentrations of CASE (Figure 1).
Figure 1. Effect of CASE on the Production of Inflammatory Mediators by RAW264.7 Cells Stimulated with LPS. Cytokine and NO Levels were Measured in the Supernatant of Cells Treated with CASE. Mean±SD of Three Independent Experiments. ‘NC’ Indicates Non-LPS Stimulated Control.
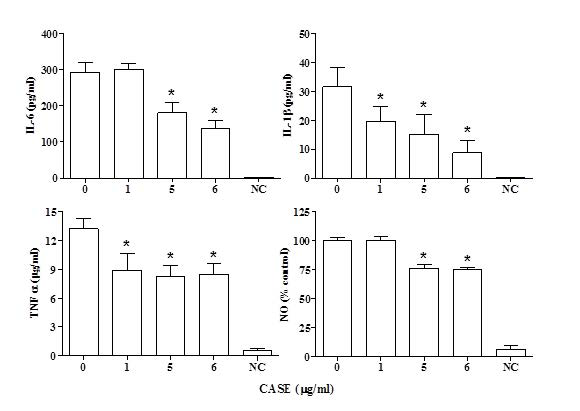
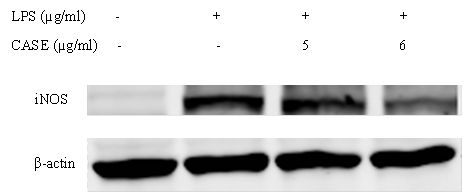
NO production by LPS-stimulated RAW264.7 cells co-treated with CASE was assessed using the Greiss reagent. CASE inhibited the formation of nitrite in a concentration-dependent manner with a significant reduction at concentrations greater than 5 μg/mL (Figure 1). Western blot analysis showed that CASE dose-dependently reduced the protein expression of iNOS in LPS-stimulated RAW264.7 cells after 24 h treatment (Figure 2).
Figure 2. Effect of CASE on the Protein Expression of iNOS in LPS-Stimulated RAW264.7 Cells. Cells were Treated with CASE for 24 h. Figure is Representative of Three Independent Experiments.
Inhibition of PGE2 Production by CASE
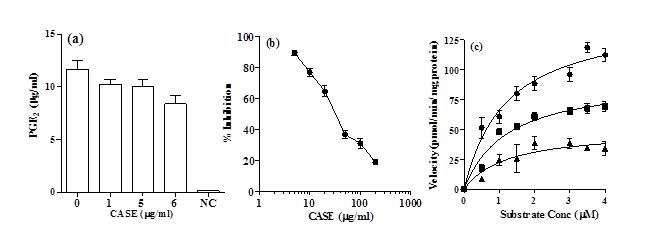
PGE2 production by RAW264.7 cells was assessed after co-incubation with 1 µg/mL LPS and CASE for 24 h. It was observed that the PGE2 production was significantly reduced by treatment with 6μg/mL CASE compared to LPS-stimulated controls (Figure 3a). Western blot analysis of COX-2 showed that CASE did not affect its expression (results not shown).
Figure 3. (a) The effect of CASE on the Production of PGE2 in RAW264.7 Cells Stimulated with LPS for 24 h. Mean±SD of Three Independent Experiments. (b) The Effect of the CASE on the Activity of Pure Secreted PLA2. Symbols Represent Mean±SD (n=5) (c) Kinetic Analysis of CASE Inhibition of Secreted PLA2. Concentrations of CASE Used were (●) nil, (■) 36 µg/mL and (▲) 72 µg/mL. Inhibitory kinetics were Determined by Using Michelis-Menton Analysis.
Inhibition of PLA2 Activity by CASE
CASE dose-dependently inhibited the activity of secreted PLA2 in a cell-free system. The IC50 of CASE was 36 µg/mL (Figure 3b).
Kinetic analysis showed that CASE significantly reduced the V-max but had no significant effect on Km of PLA2 (Figure 3c; Table 1). These results suggest that CASE inhibits PLA2 in a non-competitive manner with respect to substrate concentration.
| Table 1. Kinetic Analysis of Inhibition of Secreted PLA2 by CASE* |
|
CASE (µg/mL)
|
0 |
36 |
72 |
| Km (μM substrate) |
1.2±0.1a |
1.2±0.1a |
1.2±0.3a
|
|
Vmax (pmol/min/mg protein)
|
145.8±15.6a |
92.6±3.9b |
49.5±3.9c
|
| *Values in the same row not sharing a common superscript letter are significantly different (p< 0.05) |
Change in the Expression of NF-kB by CASE
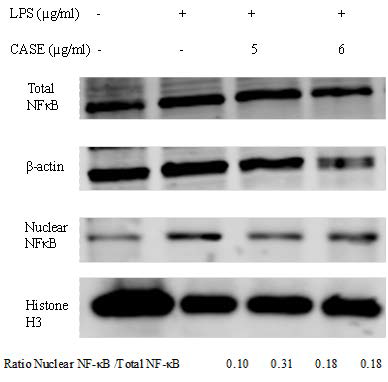
The nuclear localization of NF-kB is a critical signal in LPS-induced inflammation. Co-incubation with CASE reduced nuclear levels of NF-kB, but had no effect on total NF-kB expression (Figure 4).
Figure 4. Effect of CASE on Nuclear Translocation of NF-kB in LPS-Stimulated RAW264.7 Cells. Nuclear and Total Levels NF-kB of were Determined by Western Blot .β-actin and Histone H3 were Used as Protein Loading Controls for Total and Nuclear Protein Respectively. Representative Results of Two Independent Experiments.
DISCUSSION
This study was carried out to assess the anti-inflammatory effects of CASE in LPS-stimulated RAW264.7 murine macrophage cell line. On stimulation by LPS, macrophages produce pro-inflammatory cytokines, eicosanoids and NO. These inflammatory mediators play a key role in the progression of inflammatory diseases. Treatment with CASE reduced production of TNF-α, IL1β, and IL-6.IL-1 β induces the expression of COX-2 and iNOS. TNF-α binds to different receptors than IL-1β, the post-receptor events are similar, and both activate a similar portfolio of genes. IL-6 also triggers the formation of other inflammatory markers including eicosanoids and NO, and as a result, there is growing interest in developing anti-IL-6 agents.10 Inhibition of these cytokines by CASE indicates that the formation of downstream inflammatory products may also be inhibited.
CASE inhibited LPS-induced NO production. NO is a key inflammatory mediator and inhibition of NO production has an anti-inflammatory effect. CASE reduced the expression of iNOS providing a mechanism of the observed decrease of NO. The promoter region of iNOS gene contains several binding sites for transcriptional factors such as NF-kB and AP-1 as well as other proteins. LPS-stimulated induction of iNOS is mediated through NF-kB.11 CASE inhibited the formation of PGE2, a pro-inflammatory eicosanoid, but did not affect COX2, its biosynthetic enzyme. CASE also inhibited the activity of secreted PLA2. CASE-mediated inhibition of PGE2 production may result from inhibition of PLA2. Alternatively, it could be the result of inhibition of COX-2 activity. PLA2 catalyzes the release of arachidonic acid from phospholipids. Arachidonic acid is then metabolized through the COX pathway to prostaglandins and through the LOX pathway to leukotrienes.COX-2 is a known drug target with clinically useful inhibitors, whereas inhibitors of PLA2 are currently sought as potentially useful anti-inflammatory agents.12 Cytosolic PLA2 (cPLA2 ) is upregulated due to stimulation with LPS; however, secreted PLA2 was used in this study.
The nuclear translocation of NF-kB was reduced by treatment with CASE. NF-kB is a transcription factor, which in the resting state is bound via non-covalent interactions to inhibitor of Kappa B (IκB) and sequestered in the cytoplasm. Treatment with LPS causes phosphorylation of IκB and subsequently it degrades thus allowing NF-kB to enter nucleus and induce gene expression. NF-kB is involved in regulating many aspects of cellular function, including immune response. The expression of inflammatory cytokines, adhesion molecules, angiogenic factors COX-2 and iNOS are all regulated by NF-kB.13 NF-kB therefore represents a possible pathway through which the CASE may exert its anti-inflammatory effects. Modulation of NF-kB signaling may account for the effects of CASE on cytokine production and iNOS expression. The effects on PGE2 and PLA2, however, are likely due to an alternative mechanism, since COX-2 expression was unchanged by CASE.
CONCLUSION
This study characterized the in vitro anti-inflammatory effects of a colored extract obtained from avocado seed. The extract was found to reduce the production of pro-inflammatory mediators by stimulated macrophage cells. Anti-inflammatory medications such as NSAIDs and steroids are commonly used to treat various diseases. CASE represents a potential source for novel antiinflammatory compounds that can be developed as a functional food ingredient or as pharmaceuticals.
FUNDING
This work was supported by the United States Department of Agriculture (USDA) National Institute of Food and Agriculture Federal Appropriations under Project PEN04565.
CONFLICTS OF INTEREST
The authors Dabas, Ziegler, and Lambert have applied for a US patent on the colored compound described in this manuscript. Authors Ziegler and Lambert have an equity interest in Persea Naturals limited liability company (LLC) that has licensed related technology from Pennsylvania State University, PA, USA.









