CASE REPORT
This case report pertains to a 46-year-old male Caucasian who had endoscopic treatment for a pituitary adenona three years previously. He required replacement hydrocortisone and testosterone. Prior to the surgery, he was not known to have any other neurological issues.
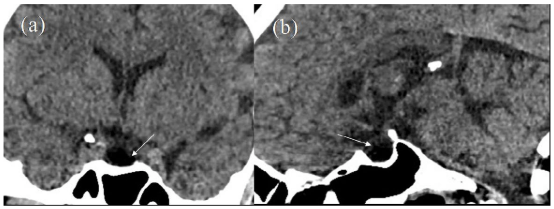
A year after surgery following minor trauma, he underwent a computed tomography (CT) scan of the head. This confirmed no residual pituitary tumour (Figure 1). He was somewhat non-compliant with endocrine follow-up. Surveillance magnetic resonance imaging (MRI) imaging 2 years following initial surgery demonstrated post-up change with some enhancing tissue scaling the pituitary stalk and hypothalamic region; this was not clearly seen on the previous CT scan, possibly due to different modalities and absence of contrast imaging. Two months later, he developed polydipsia, fatigue and a partial left-sided homonymous visual field deficit. There was progressive confusion with fluent dysphasia and inattention. He was pyrexial. Gaze evoked nystagmus was present to the right. The rest of his examination was unremarkable.
Figure 1: Initial CT Scan Performed at the Time of Head Injury. (a) Coronal and (b) Sagittal Reconstructions Show an Empty Sella (white arrows) with no Discernible Soft Tissue.
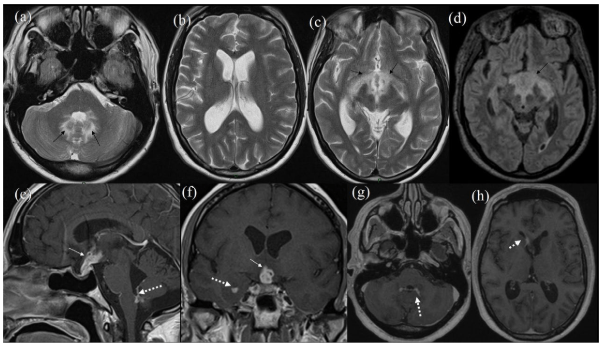
MRI brain demonstrated significant progression of the abnormalities seen on the earlier scan with increased enhancing tissue involving the pituitary stalk, hypothalamic area and floor of the 3rd ventricle, also including the optic chiasm (Figure 2). Associated signal changes/oedema was noted in the vicinity of 3rd ventricle, optic tracts, posterior limb of internal capsule bilaterally and anterior aspect of the midbrain. There was further enhancement within the 4th ventricle on both sides and associated prominent oedema in the cerebellum. In addition there was ependymal enhancement within the right frontal and temporal horns (Figure 3). The ventricular size had increased since the previous scan. This rapid growth rate (over two months) and ependymal enhancement was considered unlikely to represent pituitary tumour recurrence and the likelihood of an inflammatory aetiology or lymphoma was considered. He was given dexamethasone 8 mg twice daily which immediately reversed his confusion. At this stage the differential included an inflammatory or lymphomatous process. He became hypothyroid.
Figure 2: Initial MRI During First follow-up. Post-Contrast MRI (a) Coronal and (b) Sagittal Showing some Enhancement along Pituitary Stalk Reaching upto Hypothalamic Region (White Arrows).
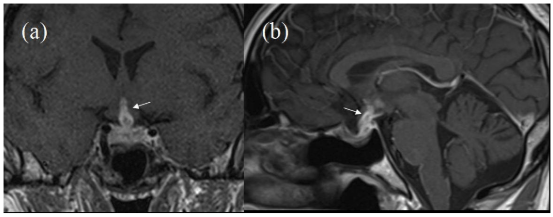
Figure 3: Follow-up MRI. T2 Axial Images (a-c) and FLAIR Axial (d). Black Arrow in (a) Showing Oedema in Cerebellum Around 4th Ventricle. Ventricular Size has Increased Since Previous Scan.Black Arrows in (c, d) Showing Oedema Along Optic Tracts, Posterior Limb of External Capule Extending Posteriorly in Midbrain. Post-Contrast T1 Weighted Sagittal (e), Coronal (f) and Axials (g, h). White Arrow in (e, f) Showing Increase in Enhancing Soft Tissue along Pituitary Stalk and Hypothalamus. Broken White Arrow in (e, g) Showing Ependymal Enhancement in 4th Ventricle. Broken White Arrow in (f) Showing Ependymal Enhancement in Temporal Horn. Broken White Arrow in (h) Showing Ependymal Enhancement in Right Frontal Horn.
The following blood tests were either normal or negative: plasma viscosity, C-reactive protein (CRP), C3, C4, anti-DNA and extractable nuclear antigen (ENA) antibodies. Spinal fluid analysis revealed a lymphocytosis of 345×106/L and a protein of 2459 mg/L. Oligoclonal bands were detected in the cerebrospinal fluid (CSF) only. Cytology and microbiology was negative.
Repeat MRI scan 11 days later showed persistence of an abnormally enhancing tissue in the hypothalamus and pituitary stalk but reduced in volume with some residual enhancement in the 4th Ventricle. The midbrain oedema had resolved when compared to the previous scan. Clinically, he improved but had residual mild impairment of gait. Dexamethasone was reduced and he was discharged on a tapering dose of oral prednisolone.
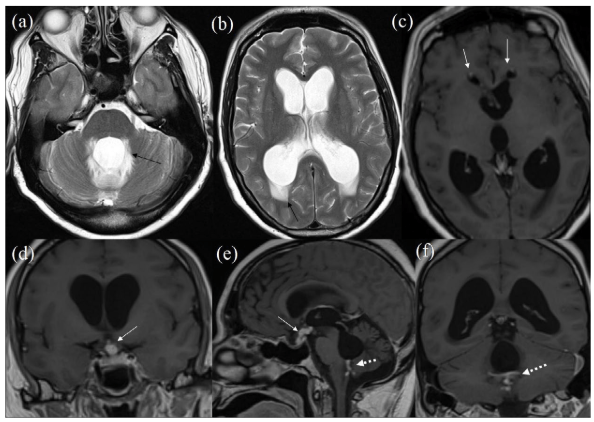
However, three months later, he was readmitted with worsening confusion. CT head revealed acute hydrocephalus, with increased dilation of the lateral, 3rd and 4th ventricles, and generalised sulcal effacement, with no midline shift. A brain MRI scan confirmed lateral, 3rd and 4th ventricular dilation. Though the abnormally enhancing tissue in the pituitary stalk and hypothalamus had slightly reduced in volume, there was obstruction at the level of 4th ventricle, probably secondary to enhancing lesions with likely adhesions (Figure 4).
Figure 4: MRI on Clinical Deterioration. T2 Axials (a, b) Showing Significant Worsening ofHydrocephalus. Black Arrows Showing Significant Periventricular Oedema. Post-Contrast T1 Axial(c),Coronal (d, f) and Sagittal (e). White Arrows in (c) Showing Subtle Residual Enhancement in Frontal Horn. White Arrow in (d, e) Showing Reduced Infundibular-Hypothalamic Enhancement. Broken White Arrows in (e, f) Showing Residual Enhancement on Fourth Ventricular Outflow whichAppears Obstructed Causing Hydrocephalus.
He underwent an endoscopic third ventriculostomy and biopsy of the floor of the 3rd ventricle inflammatory lesions.
Histopathology
Histologically there was abnormal tissue with mixed inflammatory cell infiltrate composed of small lymphocytes, macrophages and plasma cells. There were also occasionally scattered larger cells with large nuclei, prominent nucleoli and abundant eosinophilic cytoplasm. Histopathlogical analysis also showed many CD20-positive B-cells, CD3-positive T-cells, CD138-positive plasma cells as well as CD68-positive macrophages, all confirming the mixed inflammatory cell infiltrate identified on H&E staining. Markers of Langerhan’s cell histiocytosis (Langerhans, CD1a) were negative. The conclusion was an acute on chronic reactive process of unknown aetiology. There was no evidence of any neoplasia, including lymphoma or pituitary adenoma. Microbiology specimens were also sent of tissue and CSF, which were all negative. Prolonged culture was performed and there was no evidence of tuberculous disease. Similarly, testing for sarcoid, human immunodeficiency virus (HIV) and syphilis was negative.
He had insertion of an external ventricular drain, which was converted to a right frontal ventriculo-peritoneal shunt. He was discharged home (on a course of hydrocortisone) and was due to be followed-up for a repeat MRI. Unfortunately, the patient died two months later. The post-mortem identified no cause of death, though it was noted that blood alcohol levels were slightly elevated and it was postulated that he may have failed to self administer oral hydrocortisone.
DISCUSSION
The current case is complicated by presence of a previously excised benign pituitary adenoma, confirmed on re-analysis of the original histology. The adenoma appeared to have been adequately removed as the initial post-trauma CT confirmed that no definite mass was present.
The patient’s presentation to us was thought to be due to an inflammatory process predominantly affecting pituitary-hypothalamic axis with further ependymal involvement at several places that subsequently resulted in obstructive hydrocephalus.
The differential diagnostic possibilities here include infection, inflammation and neoplasia.
Regarding infection; tuberculosis (TB) was considered but culture and biopsies were negative. Radiologically the features of langerhans cell histiocytosis (LCH) were initially thought consistent. Other differential diagnoses include lymphocytic hypophysitis (LYH), sarcoidosis and Erdheim-Chester Disease. The latter which would correlate with the presence of high lymphocytes in the CSF and response to steroids. Regarding sarcoidosis this was contemplated and indeed the CSF findings could be compatible. In sarcoidosis there is often a lymphocytosis with high CSF protein. Oligoclonal banding may also be present. We did not however demonstrate non-caesiating granuloma on histology. Acute disseminated encephalomyelitis (ADEM) and multiple sclerosis were given a thought though dismissed since imaging characteristics did not support this diagnosis. Similarly, there was no histological features of vasculitis seen.
Before histology, a serious consideration was given to LCH. Intra-cranial LCH lesions exist in two different forms: hypothalamic-pituitary axis change (HPA-LCH), being the most common, and less frequently neurodegenerative change (ND-LCH). In HPA involvement, central diabetes insipidus (CDI) arises in up to 50% of cases,1 and anterior pituitary hormone deficiencies in 20%.2 CDI is rare and characterised by polyuria, polydipsia and hypotonic urine due to antidiuretic hormone deficiency and, whilst LCH is a cause, it is by no means the most common cause.3,4 Conversely, the latter pattern presents with a mixture of ataxia, nystagmus, dysarthria, spastic tetraparesis, pseudobulbar palsy or cognitive/behavioural alterations5 – the first two and latter symptoms were seen in our patient but not CDI. HPA-LCH presents on MRI with a thickened hypothalamus, thickened pituitary stalk and absent posterior pituitary bright spot. In patients presenting with diabetes insipidus.6,7 ND-LCH affects the cerebellum, basal ganglia and pons. MRI can show bilateral signal changes and non-enhancing lesions in the dentate nucleus, often extending to the surrounding white matter involving the midbrain, pons, globus pallidus and cerebellar peduncle or presenting with cerebellar atrophy.8,9,10,11,12
There are varying opinions on the indication for HPA biopsy in terms of the size of the lesion or severity of symptoms.13 Studies by Jian et al13, Leger et al14 and Adani et al15 all propose different cut-offs for biopsy, such as presence of an endocrinopathy, radiological changes (e.g., when the stalk is >7 mm) and/or clinical deterioration.
In the past, electron microscopy was used on these biopsy specimens to detect the Birbeck granules that were characteristic of the LC; however this has been replaced by the use of immunohistochemistry to detect CD207 (Langerin) or CD1a expression. CD207 is a type II transmembrane C-type lectin associated with the formation of Birbeck granules.16 This test is now the Gold Standard in the correct clinical setting.17,18
Erdheim-chester disease (ECD) is a rare disease, frequently seen in adults. It shows infiltration of a wide variety of tissues by cells of macrophage and histiocytic lineage, considered distinct from LCH. It is an infiltrative process with preponderance of lipid-laden histiocytes mixed with lymphocytes and eosinophils. It can vary from a focal abnormality to an extensive multi-organ and life-threatening condition. It can show endocrine manifestations including CDI, as well as hypopituitarism and hyperprolactinaemia. However, on MRI, pituitary mass lesions or stalk thickening is rarely found although normal T1 high signal is frequently absent from the posterior pituitary. ECD was considered but excluded due to lack of typical findings and lack of systemic involvement.19 LYH or autoimmune hypophystis is characterised by focal or diffuse inflammatory infiltration and variable degree of pituitary involvement.19 It can be divided into three subtypes: lymphocytic adenohypophysitis, lymphocytic infundibuloneurohypophysitis and lymphocytic panhypophysitis. It can be frequently associated with other autoimmune conditions, most commonly autoimmune thyroid disease (reported in 15-25% of LYH cases, 75% of these being chronic autoimmune thyroiditis). Autoantibodies to various cells can be found, including prolactin, GH and Adrenocorticotropic hormone (ACTH) secreting cells. In our case, due to previous pituitary surgery, assessment of these was not thought to be of use. Histopathology is the gold standard for diagnosis of LYH, the characteristic feature being a diffuse polyclonal lymphocytic infiltrate with predominance of T-cells, particularly CD4 cells. Clinically, it can present with headache, impaired vision, nausea, vomiting, fatigue, weakness, anorexia, and visual disturbances. There can be partial or total hypopituitarism.19
Sarcoidosis is a multisystem granulomatous disorder.20 Neurosarcoidosis occurs in about 10% of affected patients. The disease primarily involves leptomeninges and extends along perivascular spaces resulting in parenchymal lesions. The lesions have predilection for basal structures of brain, including hypothalamus and pituitary gland. Therefore involvement of hypothalamic-pituitary axis (resulting in CDI and anterior pituitary failure) is the most common feature of neurosarcoidosis. On MRI, the lesions are seen as infiltration of the dura mater which is thickened and show enhancement on contrast, also involving infundibular stalk, optic chiasm and floor of third ventricle that can be quite thickened, sometimes with mass like areas.20
CNS lymphoma is another entity that can be primary or secondary. It could clearly have been a case of either of these, although primary more likely due to absence of systemic disease. These can be steroid responsive initially. However, histology was not supportive. On reflection, amongst all possibilities, the imaging findings were considered closest to LCH although disproved on histolopathology. The histology itself was more in favour of a LYH but the inflammatory deposits seen on MRI imaging were too extensive for this condition in isolation. Therefore, there was absence of clear similarity with any of the known conditions affecting this region, leading us to conclude that this was a case of steroid responsive inflammatory process of unknown aetiology.
The patient was treated with steroids and subsequent MRI did prove that there was improvement in hypothalamic/infundibular lesion. However, the ependymal involvement was associated with adhesions that led to 4th ventricle outflow obstruction and acute obstructive hydrocephalus with clinical deterioration. While this was noted earlier, this continued to progress despite the use of steroids. This clearly shows that depending upon the site of involvement, a steroid responsive inflammatory condition can still have adverse outcome. Those with involvement of the fourth ventricle and potentially the sylvian aqueduct should be closely followed with early imaging, to detect hydrocephalus.
It is worth noting that this patient did not die of acute hydrocephalus and large inflammatory lesions within the brain were not seen on post-mortem examination. The cause of death is unknown, but not thought to be directly related to the disease process in question.
CONCLUSION
We present an unusual case, complicated by previous surgery, but clearly inflammatory in origin predominantly affecting the suprasellar region but extending elsewhere to involve ependymal lining. This case highlights the fact that despite extensive literature and rather well addressed reviews available, it is possible to have a rare inflammatory condition, that can mimic other inflammatory conditions such as LCH or LYH in different ways, yet probably represents another entity not yet fully characterised. It also highlights that despite the steroid responsive nature, the ependymal involvement can result in progressive acute obstructive hydrocephalus with clinical deterioration, thereby suggesting close follow-up and early imaging for early detection and treatment of this complication.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of intertest.
CONSENT
Written informed consent was obtained from the patient for publication of this case report and any images.









