INTRODUCTION
As the number of genetic tests for multifactorial diseases continues to grow in clinical settings, individuals and family members at risk for these conditions are facing more decisions regarding current and emerging tests.1 In many instances, undergoing genetic tests requires complicated psychological and behavioral adjustments.2,3 The current literature on psychological factors and decisions to undergo genetic testing has heavily centered on 1) cancers as the disease focus, such as hereditary breast, ovarian and colon cancers,4,5 2) single-gene disorders with identified etiology, such as Down syndrome and cystic fibrosis,6 and 3) the psychological and behavioral impact of genetic testing.7 Less attention has been paid to the psychological determinants associated with decisions to undergo genetic testing for complex neurodevelopmental disorders – autism spectrum disorders (ASD), in particular.
ASD is a range of conditions characterized by social impairment, communication difficulties and restricted, repetitive behaviors.8 Strong evidence suggests that ASD is among the most heritable of all neurodevelopmental conditions.9,10 Parents of children with ASD are at an increased risk of having another affected pregnancy.8,11 The recurrence risk of ASD is between 2-9% if one child was diagnosed with ASD, and 25-35% if two or more affected children were identified in one family.9 Studies have suggested monozygotic twins are much more likely to have ASD than dizygotic twins. Similarly, other chromosomal abnormalities are more prevalent in families and relatives with ASD.11,12
Current Genetic Tests Recommended for ASD
Until recently, there has been no single laboratory test, such as the BRCA gene test for breast cancer and FMR1 screening for fragile X,13,14 which can be used for diagnosing ASD exclusively. The available clinical genetic tests recommended for identifying the etiology of ASD vary by medical authorities. The American Academy of Neurology and the American Academy of Pediatrics recommend traditional cytogenetic tests including G-banded karyotyping and Fragile X screening in patients with ASD.15,16 Recently, Chromosomal Microarray technology (CMA) has emerged as a more powerful tool providing higher resolution and better diagnostic yield than traditional tests.17 Both the American College of Medical Genetics and Genomics (ACMG) and the International Standard Cytogenetic Array Consortium recommend CMA as the first-tier test for people affected with ASD.18
Generally, the potential benefits of autism genetic testing encompass identifying the causes of ASD, promoting early detection and developing treatment plans.19 As noted by ACMG clinical guidelines (2013 revision), “using current knowledge and technology, a thorough clinical genetics evaluation of patients with ASD is estimated to result in an identified etiology in 30-40% of individuals.”20(404) However, similar to other genetic tests, autism genetic testing might also involve a number of ethical, legal and social implications, such as genetic discrimination and insurance concerns.21
Decisions Regarding Undergoing Autism Genetic Testing
The decisions to undergo genetic testing for specific conditions might vary widely among at-risk populations.4 Previous literature has shown that the uptake of genetic tests was likely to be predicted by a number of psychological factors, such as higher perceived disease risk, greater level of anxiety over the disease or desire for emotional relief.4,22 Unfortunately, psychological factors associated with decisions regarding testing for ASD are largely unknown in the existing literature. Decisions to undergo autism genetic testing can be more complicated than other conditions due to the following reasons: 1) The multifactorial nature of ASD (with more than one single gene involved), 2) the relatively low detection rate with the current technology (compared with single-gene disorders, such as Down syndrome and cystic fibrosis), 3) the inability to test for disease severity,23 and 4) lack of evidence for clinical utility.23,24 Due to these test constraints, ASD-affected people, their families, and at-risk populations might experience a host of unique psychological factors associated with the decision to undergo genetic testing for ASD.25,26
In addition to the psychological burden, cultural identity might also shape the decisions whether to undergo the test for ASD. To date, little is known on this specific test decision within Asian populations, both within and outside the US. To ensure the appropriate use of genetic technology and reduce the concerns regarding autism genetic testing, it is critical to understand the psychological factors that determine the test uptake among parents of children with ASD. This study will specifically investigate the psychological factors that determine the test decisions among a sample of Taiwan parents of children with ASD. Understanding the decisions regarding autism genetic testing is expected to have a broader impact in Taiwan, primarily due to 1) the misuse of genetic testing in that country, 2) the societal pressure or stigmatization for having children with mental disorders, 3) lack of policies or official guidelines to regulate autism genetic testing.
This study will examine the attitudes and decision making regarding autism genetic testing among parents of children with ASD. Specifically, we aim to 1) propose an integrated theoretical model for undergoing ASD genetic testing, and 2) examine the psychological factors, attitudes and intention regarding autism genetic testing among a sample of parents with autistic children in Taiwan, based on our proposed theoretical framework.
THERORETICAL FRAMEWORK
Based on the current literature on factors determining the attitudes, beliefs and decision making regarding genetic testing,27,28,29 We propose a theoretical model (See Figure 1) for this study. This model contains three key constructs, i.e. affect-type variables, attitudes and intention from the following validated theories: the Theory of Planned Behavior (TPB),30 Self-regulation theory (SRT),31 and the Transactional Model of Stress and Coping (TMSC).32
Figure 1: Theoretical model of parents’ intention of undergoing ASD genetic testing.
A: Attitudes toward testing the affected child and the family members
B: Attitudes toward carrier, prenatal diagnosis and newborn screening
C: Attitudes toward testing individuals with family history of ASD
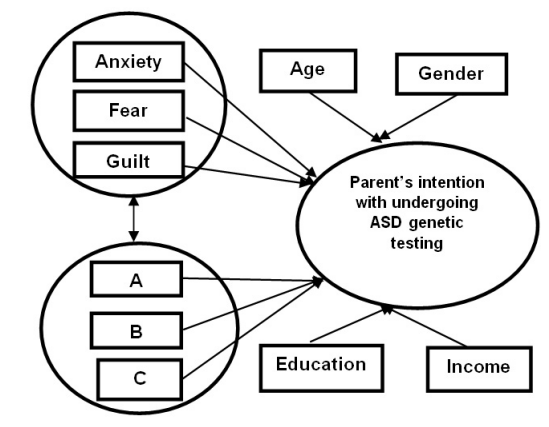
The model we proposed is specifically designed to explain the emotional factors that facilitate or inhibit parents’ decisions to undergo autism genetic testing. The underlying reason for constructing this combined model is as follows. Although preeminent health behavioral theories, such as the Health Belief Model (HBM) and the TPB,33 have been widely employed in examining the decision-making processes related to genetic testing, they lack an important component in predicting the intention or the behavioral change: emotions.28,34
Although less frequently adopted for genetic testing research, the two health psychology theories, SRT and the TMSC, have been validated and used to explain how individuals might exhibit emotional responses, such as stress and fear, and how they might cope with the emotional distress.28 In this study, I propose to add emotional factors as influences on the decisions regarding autism genetic testing among parents of children with ASD.
The salient features of the proposed model include the emphasis on emotional appraisal, coupled with attitudinal factors in assessing parents’ decision-making processes. This model highlights the affect-type variables as important constructs to explain how people’s decisions regarding genetic testing/screenings might be shaped.
The key outcome variable in the model we propose is parents’ behavioral intention of undergoing ASD genetic testing. Affect-type variables and attitudes are the two predictor variables (Figure 1). It is hypothesized that in this model, parents’ intention regarding ASD genetic testing is correlated with their emotional responses and attitudes toward the test. Each of the three key variables (i.e. affect-type variables, attitudes and intention) interacts and connects with each other. The affect-type variables are composed of three subdomains: anxiety, fear and guilt. Anxiety has three subgroups: trait anxiety, anxiety caused by ASD and anxiety caused by ASD genetic testing. Based on past literature,29,35,36 the parental attitudes are also further divided into three subdomains: a) attitudes toward testing the affected child and the family members, b) attitudes toward carrier testing, prenatal diagnosis and newborn screening, and c) attitudes toward testing individuals with family history of ASD. Meanwhile, overall moderating factors such as gender, age, and income might influence these aforementioned factors. Below we will contextualize each of the variables employed in this model.
Affect-Type Factors
Based on the preliminary findings from our previous work on parents’ attitudes toward autism genetic testing and the reasons listed below, we specifically intend to test three emotional variables: i.e. fear, anxiety and guilt.
Fear: Both SRT and the TMSC delineate fear as an important predictor in making decisions when people experience a specific health situation.28 Fear is among the most studied emotion in social science, and can be a strong motivator for actions.37 As Protection Motivation Theory (PMT) theorizes, “fear may be considered a relational construct, aroused in response to a situation that is judged as dangerous and toward which protective action is taken”.38(p51) Evidence shows that stronger levels of fear can induce greater changes in attitudes, intentions, and behaviors.39 In this study, we will test the specific kind of fear related to the negative consequences, privacy issues, genetic stigma and discrimination caused by ASD genetic testing. Because our sample was from Taiwan, parents’ perspectives could possibly be influenced by traditional Chinese culture and societal pressure on “birth defect”. From the perspectives of traditional Chinese culture, children with “birth defect”, especially those with mental disorders might be stigmatized and excluded from the mainstream society.40
Anxiety: Although anxiety is not specifically described as affect-type variables in the constructs of SRT and the TMSC, previous literature has extensively described anxiety as one of the most common emotional response related to genetic testing.5,41 Anxiety had two related types: state anxiety and trait anxiety. State anxiety is a transitory emotional reaction which includes a subjective feeling of nervousness, tension and worry.5 However, trait anxiety refers to an enduring characteristic of a person that can be used to explain a person’s behavioral consistencies, and determines the likelihood a person will experience anxiety in stressful situations.42 Previous studies have demonstrated that both state anxiety and trait anxiety were related to the uptake of genetic testing for hereditary breast, ovarian and colon cancers.5 In this proposed study, both trait anxiety and state anxiety will be evaluated.
Guilt: Compared with anxiety and fear, feeling of guilt is not well researched in genetic testing research. However, guilt is a prevalent emotional response to hereditary diseases across a wide range of genetic conditions.43 In the context of genetic screenings, feeling of guilt can be caused by feeling the passing of a faulty gene to children, causing them to have certain genetic disease or the higher risk for developing the disease. Previous study indicates that the emotional responses to genetic conditions are often characterized by feelings of guilt.43 Because genetic testing is meant to detect certain diseases running in the family, it is very likely to provoke the feeling of guilt among family members and affect their individual intention of undergoing genetic testing. We will specifically assess guilt associated with having children with ASD and undergoing genetic testing for ASD in this study.
Attitudes: According to Eagly and Chaiken,44 attitude is defined as the subjective evaluation of an object or action and it can be positive or negative. Attitude is a major determinant elucidated in TPB associated with people’s engagement in a specific behavior.30 Prior studies that explore the domains of TPB show individuals’ positive attitudes are correlated with their intentions to be tested for colorectal, breast/ovarian cancer and Alzheimer’s disease.45,46,47 Two dimensions of attitudes can be measured: values and beliefs.48 This study will assess both dimensions.
Intention: As depicted in the TPB, behavior is directly driven by people’s intention. TPB has been substantially used to predict and explain human behavior in diverse health-related contexts including genetic testing intentions.33 Similar to the TPB, the Model of Interpersonal Behavior (MIP) also emphasizes the main construct of “intention” as the antecedent of individuals’ behavior. The intention to be tested in this study is the intention of undergoing genetic testing for 1) children with ASD, 2) siblings of children with ASD, 3) themselves, 4) their spouses, 5) relatives from their biological family, and 6) relatives of their spouses.
MODERATORS
As the HBM, CSM and TMSC theorize, a variety of social-demographic factors might modify how individuals perceive health issues or concerns.28 These factors include variables such as age, education, socioeconomic status and ethnicity or religious beliefs.
For instance, interest in and uptake of genetic testing for hereditary cancer has been associated with having more years of education, a higher income, a higher education level and better health insurance coverage.49 In this proposed study, age, gender, educational level, and annual household income will be measured as moderating factors.
METHODOLOGY AND STUDY DESIGN
Study Design
This proposed empirical study is part of a larger research project initiated by Dr. Lei-Shih Chen in the Department of Health and Kinesiology at Texas A & M University and co-directed by Dr. Tse-Yang Huang in the Department of Special Education, National Hsinchu University of Education. The research project, funded by the Chiang Ching-kuo Foundation for International Scholarly Exchange, was conducted among parents of children with ASD in Taiwan. The entire project was completed in three phases during September 2012 and January 2013: survey development, pilot test and survey distribution.
Altogether, we surveyed almost 250 schools and sent 862 surveys to parents of children in diverse autism communities in Taiwan. The response rate was 52.8%. The research protocols were approved by Texas A & M’s institutional review board. More details were provided in our previously published work.50
Measures
The measures used in this analysis included two primary categories, i.e., the outcome variable and predictor variables. The outcome variable is parents’ intention regarding the test uptake. Parents’ intension was measured by six items scaled on a 4-point response format. Both emotional responses and attitudes were predicator variables in this study. Anxiety, fear and guilt are the three components of emotional responses. Each of them was assessed by items in a 4-points response format.
Data Analysis
First, we performed descriptive and exploratory analyses with the assistance of IBM SPSS version 22. The psychometric properties of both predictor and outcome variables were examined before conducting path modeling and factor analysis. Statistical significance of hypothesis tests is typically reported using a type I error rate of 0.05. Actual p-values were provided whenever possible.
Second, we applied a two-step structural equation model analysis to assess whether the data supported the hypothesized model. The initial step included a Confirmatory Factor Analysis (CFA) to determine latent constructs in this model. In this first step, we performed a group of confirmatory factor analyses to measure measurement models on the following latent variables: 1) trait anxiety, 2) state anxiety caused by ASD, 3) state anxiety caused by ASD genetic testing, 4) guilt carrying the ASD genes (Guilt 1), 5) guilt caused by undergoing ASD genetic testing (Guilt 2), 6) attitudes toward testing the immediate family members (Attitude A), 7) attitudes toward carrier testing, prenatal genetic testing, pre-implantation genetic disorders and newborn screening (Attitude B), 8) attitudes toward testing ASD-affected children (Attitude C), and 9) intention. Measuring latent constructs allows us to assess if measurement error related to each construct exited. The measurement analysis also provided diagnoses to the validity of the proposed constructs.
After we established an adequate fit for the measurement model with theoretical considerations, we developed a structural model to evaluate the interactions between and among these proposed variables, i.e. anxiety and intention, fear & guilt and intention as well as attitudes and intention. The entire measurement process was assisted by Mplus MLR estimator. We used Chi-square, Comparative Fit Index, the Root Mean Square Root Error of Approximation (RMSEA) and Standardized Root Mean Residual (SRMR) to evaluate the model fit.51
RESULTS
Sample
The final sample included 334(77.5%) females and 97(22.5%) males. They were all parents of children with ASD. The majority of the participants (95.2%) were born in Taiwan and 67.3% (n=293) of the parents claimed they had never been to college. Slightly more than half of the participants (n=218) claimed that they did not have a full-time job. More than one fourth of the parents (33.6%, n=143) reported that their annual household income was less than $20 k.
Preliminary Analyses
We used the most widely used technique for estimating SEM, Full Information Maximum Likelihood (FIML), to deal with the missing date. The amount of missing data ranged from 2% to 7.6%. Under the assumption of multivariate normality, FIML produces parameter estimates that “have optimal large-sample properties: consistency, asymptotic efficiency, and asymptotic normality”.52(p289) Followed the procedure,53 we imputed -99 to replace the missing values in the dataset.
Based on Mardia’s measure of relative Multivariate Kurtosis (MK),54 we tested the normality of the variables and moderators (emotional factors, attitudes, and intention, as well as age, gender, education income and religion). The skewness and kurtosis coefficients ranged from +1 to -1, indicating no violation of the normality assumption.
Confirmatory Factor Analysis
Correlation and reliability: We established the correlation matrix for the items related to emotions, attitude and intentions as well as participants’ demographic information. The individual items comprising emotional and attitudinal factors primarily correlated among themselves. For instance, the highest correlation was found between the items for Attitude A and Attitude B (r=.679, p<0.01). ASD anxiety and trait anxiety (r=.623, p<0.01) were highly correlated also. In addition, the correlations between fear and guilt were significant (r=.442, p<0.01), albeit not as high. These results provide support for the existence of the hypothesized latent constructs proposed in this study.
Construct validity: Because our model involved four latent variables, it was important to first establish measurement adequacy before testing the structural relationships proposed in Figure 1. We conducted a series of confirmatory factor analyses (CFAs) to evaluate the factorial validity of the measurement scales used in this study. All factor loadings were significant at the .001 level.
Our results showed that, as we hypothesized, the measurement items testing “trait anxiety” loaded on one factor (range of factor loading: 0.582-.0754; 6 items), ASD-related anxiety loaded on one factor (range of factor loading: 0.762-0.850; 6 items) and GT anxiety-anxiety caused by ASD genetic testing also loaded on one factor (range of factor loading: 0.853-0.929, 5 items). Similarly, all five items regarding “fear” were loaded on one factor (range of factor loading=.80). In addition, among the nine items for “guilt”, the first three items – Guilt 1 loaded as one factor (range of factor loading: 0.596-0.924, three items), and the six remaining items-Guilt 2 loaded as a second factor (range of factor loading=0.776-0.874). The three items for Guilt 1 refer to the feeling of guilt brought by passing the ASD-associated genes to the family members; the remainders represented Guilt 2, which mainly discussed the feeling of guilt about taking the immediate and extended families to undergo autism genetic testing.
Factor loading also supported our hypotheses in dividing the “attitude” items into three categories. These three categories included Attitude A: Attitudes toward testing the affected child and the family members; (range of factor loading=0.862-0.900; 5 items), Attitude B: Attitudes toward carrier, prenatal diagnosis and newborn screening (range of factor loading: 0.773-0.853, 6 items) and Attitude C: Attitudes toward testing individuals (range of factor loading: 0.580-0.899, 3 items).
Although all these scales were all adopted from previous literature, the initial CFAs of the intention scale (1-6) showed two items form intention did not load on the latent construct at an acceptable level (i.e. with loading below 0.45). Based on this finding, we removed these two items from the scale. The range of the factor loading for the remaining four items was 0.736-0.912.
A subsequent CFA containing all four constructs showed that the latent construct “anxiety”, “fear-&and-Guilt”, “attitudes” and “intention” and their observed measures were well supported. The resulting CFA fit statistics included a chi-square=2803.9, df.=1112, p<.001, CFI=.92, RMSEA=0.04, 90% Confidence Interval (CI) for RMSEA [0.043 0.049]), SRMR:0.06. All fit indexes fell within acceptable ranges and all the factor loading are significant (>0.7). Using Cronbach’s alpha co-efficiency, we also identified that the internal consistency of these observed items were supported.
Structural Model
After confirming that the measurement model exhibited appropriate fit, we performed SEM analyses to evaluate whether the data substantiated the hypothesized model. Latent variable Anxiety expresses parents’ tendency to experience anxiety, state anxiety caused by ASD and state anxiety caused by ASD genetic testing. Latent variable fear-&-guilt was predicted by items depicting parents’ fear about the social or legal implications caused by ASD genetic testing, guilt caused by passing down the genes associated with ASD and guilt associated with undergoing genetic testing for ASD. Latent variable Attitudes were predicted by three kinds of attitudes which included attitudes toward testing the immediate family of the affected children, attitudes toward carrier, prenatal testing, pre-implantation genetic diagnosis and newborn screening. Latent variable Intentions reflect the likelihood that parents might bring their child with ASD, bring their children without ASD, themselves, and their spouses to undergo ASD genetic testing.
A Model Modification Method
The modifications of the model were made by removing insignificant paths to improve the overall model fit.53 Additionally, reduced models were constructed to test the hypotheses after defining the final model. Both theoretical and statistical criteria were considered to evaluate the simplification of the full model in Figure 1 into a reduced, more parsimoniously alternative model. To eliminate a variable or a latent construct, we need to consider theoretical merits as well as statistical properties consideration simultaneously.
The final result indicated that the model fitted the data well: chi sq=2224.263, d.f.=1109, p<.001, CFI:0.917, SRMR:0.06, RMSEA:0.048, 90% confidence interval (CI) for RMSEA [0.043 0.049]. These results provide support for the existence of the hypothesized latent constructs proposed in this study.
CONCLUSION
To be best of our knowledge, this is the first theory-driven study that examined emotional and attitudinal predictors of the intentions to undergo ASD genetic testing among parents of children with ASD in Taiwan using SEM modeling technique. Our findings extend existing literature on decision making about undergoing genetic testing for ASD in two ways. First, we used an integrative model and SEM analyses to understand how emotions and attitudes might influence parents’ intentions to undergo ASD genetic testing. We added affect-type variables, a largely overlooked factor in genetic testing decisions, as key constructs in our proposed model. A second way that our findings contribute to the existing literature for genetic testing is by having direct implications for public health genomics education and practice. The proposed model suggests that, educational interventions might be important based on the identified relationships among the factors. Although our sample did not allow us to generalize to the entire Taiwan autism populations, our study provided support to the need of pre-test counseling and genetic education among ASD-affected populations in Taiwan.
Albeit our study was among the first to apply structural equation modeling in the field of psychological analyses in parents’ test intention associated with autism genetic testing, we realized that we need to pinpoint both the pros of cons of applying SEM in similar studies.
Pros of using SEM: The reasons that we chose SEM data analysis to explore the decisions associated with ASD genetic testing are as follows: 1) SEM is a multivariate analytical technique designed to test theoretical models with exploratory nature, since, compared to people’s decision related to cancer-related genetic testing,55,56 for instance, breast cancer and ovarian cancer, genetic testing for autism was far less understood in previous studies. 2) SEM can capture the complexity of the social science phenomena more accurately.53 Testing the theoretical constructs can make contributions to advancement of the field of health behavioral researchas we require theory-based programs. In the proposed model, SEM allows for testing and clarifying the dynamic relationship and interactions among multiple constructs, i.e. affect-type variables (anxiety, fear and guilt), attitudes and intention. 3) SEM is advantageous in controlling for the inflation of experimental (Type I) error, which potentially reduces the chance of falsely rejecting the null hypothesis.57 4) Unlike path models that only involve observed variables, SEM is compatible for both observed and latent variables, thus it can simultaneously test the measurement hypotheses (i.e., whether observed variables are good indicators of underling factors) and structure relations (i.e., whether there are direct or indirect causal effect among latent factors) in a single model.57,58
Cons of SEM: The findings of this study should be interpreted in light of the following limitations of SEM. First, although our model indicted relatively good fit indices, we cannot purely rely on model fit because the fit measurements might not relate to the predict nature of the model. Second, for any of the given SEM model, alternative models which are equivalent regarding the indexes for overall model fit, for instance, the three different kinds of anxiety can be possibly combined into one latent variable, but this might produce largely different explanation of our empirical data. Third, sample size needs to be considered adequately in estimating and interpreting the results of SEM. As indicated by Hair and colleagues,59 the estimated number for a critical sample size that would meet with the requirement for maximum likelihood estimation is 200, with above 500 being “too sensitive” because it might detect too many differences.60,61 The bare minimum for each estimated construct is ten observations. In the hypothesized model, we need to present a minimum of 190 observations.60 This study incorporates the responses from 444 parents of children, which is sufficient to detect model fit without becoming “too sensitive”.61 Therefore, it is recommended that the results from the SEM analyses need to be interpreted with caution when applied to further research in this area.
Implications and Future Research
Our study provides useful insights into genetic literacy, psychological practice, and the potential for intervention that can help move the field forward in terms of improving the diagnosis and treatment of ASD. More specifically, for parents of children with ASD, our study will assist them in understanding their emotional responses related to the use of ASD genetic testing. For health professionals, our study could help them be aware of and sensitive to parental emotional status prior to taking their children to undergo ASD genetic testing. For policy-makers, our study can aid in the creation of relevant guidelines and regulations to ASD genetic testing.62
Our study indicated that certain negative emotions (such as fear and guilt) might be the barriers for them not to undergo ASD testing. These findings reflected an urgent need to build empathetic, caring, trusting professional relationships and consider emotional factors when performing genetic counseling among parents of children with ASD. In addition, our study found it is imperative to provide community-based, educational interventions related to ASD and genetic testing, particularly in places where genetic disorders, such as Down syndrome and autism, are considered as stigma. Also, future research need to explore how perceived risks, perceived benefits, and severity levels might also play in the model and see if they might influence parents’ intention to undergo the test.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest with the information presented in this manuscript.
ACKNOWLEDGEMENT
This present study is a secondary data analysis of a large research project directed by Dr. Lei-Shih Chen (PI) in the Department of Health and Kinesiology at Texas A & M University, U.S.A and Co-directed by Dr. Tse-Yang Huang (Co-I) in the Department of Special Education, National Hsinchu University of Education, Taiwan. The research project was funded by the Chiang Ching-kuo Foundation for International Scholarly Exchange.






