INTRODUCTION
Peroxisome proliferator-activated receptor gamma (PPARg) is a transcriptional factor involved in adipocyte differentiation and insulin sensitization.1,2 It forms a heterodimer with retinoid X receptor and binds to peroxisome proliferator responsive elements in the promoter of target genes and regulates their expression.3,4 The thiazolidinedione derivative (TZD) pioglitazone is a high-affinity ligand of PPARg and clinically useful insulin sensitizer.5 However, pioglitazone therapy is associated with adverse effects such as weight gain, edema, and fluid retention; therefore, new agents that activate PPARg with less adverse effects are expected.
Recently, it was reported that the Angiotensin-II receptor blocker (ARB) telmisartan6-17 and sulfonylurea glimepiride act as ligands of PPARg18,19 and their clinical usefulness in this context has received some attention, although characterization of these new PPARg ligands has not been fully clarified. Crystal structure of PPARg binding domains revealed a large binding pocket, which may explain the diversity of PPARg ligands.20,21 Indeed, some studies showed that telmisartan bound to different sites of PPARg from TZDs, and recruited coactivators/corepressors in different manner from TZDs.6,7,8,20 Although the characterization of glimepiride has not been clearly determined, glimepiride has been reported to have extra pancreatic effects or actions of improving insulin sensitivity as well as stimulatory effect on islet beta-cells.22,23 The extrapancreatic effects of glimepiride would be partly originated from activation of PPARg. Therefore, interactions of these ligands should be studied to determine their metabolic efficacy for the development of new therapeutic strategies against insulin resistance and type 2 diabetes. Namely, combination therapy of telmisartan with glimepiride might enhance PPARg activity without adverse effects caused by TZDs.
We investigated additive and antagonistic effects among combinations of PPARg ligands such as pioglitazone, telmisartan, and glimepiride on transcriptional activity of PPARg, and speculated the efficacy of combination therapy by these agents in clinical use.
MATERIALS AND METHODS
Materials
Pioglitazone was a kind gift from Takeda Chemical Industries (Osaka, Japan). Telmisartan and glimepiride were kind gifts from Boehringer-Ingelheim (Tokyo, Japan) and SanofiAventis Pharma (Tokyo, Japan), respectively.
Plasmids
The human RXR expression plasmid pCMX-RXR, human PPARg expression plasmid pCMX-PPARg, and Luciferase (Luc) reporter gene containing the tk promoter fused to three copies of the PPRE derived from rat AOX, (tk-[PPRE]×3-Luc), were described elsewhere.24 GAL4 responsive reporter tkMH100(UAS)×4-Luc and the chimeric receptor GAL4-PPARg were also previously described.24 The expression plasmid SRC-1 fused with GAL4 DNA binding domain GAL4-SRC-1 was described elsewhere.24,25
Cell Culture and Transient Transfection
The monkey kidney cell line CV1 was grown in DMEM containing 10% fetal bovine serum, penicillin G (100 U/ml), and streptomycin (100 mg/ml) at 37 ºC under 5% CO2 . CV1 cells were trypsinized and seeded at a density of 2×105 cells/ well in 6-well plates. Using the calcium phosphate technique, the cells were co-transfected with receptor of GAL4-PPARg and tk-(MH100)×4-Luc reporter gene. In the other experiment, the CV1 cells were also co-transfected using the same method with receptors of pCMX-PPARg and PPRE-Luc reporter gene. The total amount of transfected plasmid was adjusted to 2 µg/well by adding empty vector (pCMX). After transfection for 20 h, the medium was replaced with fresh DMEM containing 10% Dextran-coated charcoal-stripped (DCC) fetal bovine serum (10% DCC serum) in the presence or absence of ligands. After 24 h incubation, the cells were harvested and Luc activity was measured. Transfection efficiencies were normalized by galactosidase assay. For interassay control, we performed transfection with Rous Sarcoma Virus (RSV)-Luc (20 ng/well), the magnitude of which was adjusted to 100.
Mammalian Two-Hybrid Assay
Using calcium phosphate technique CV1 cells in 6-well plates were co-transfected with 800 ng Luc reporter gene containing tk-(MH100)×4 promoter, 1 µg of ß-galactosidase expression plasmid, 900 ng of expression plasmid for hPPARg fused with VP16 transactivating domain, and 360 ng of plasmid for SRC-1 fused with GAL4 DNA binding domain. pCMX was added to adjust the total DNA amount. After incubation for 24 h, the cells were harvested and Luc activities measured and normalized with ß-galactosidase activity.
Statistical Analysis
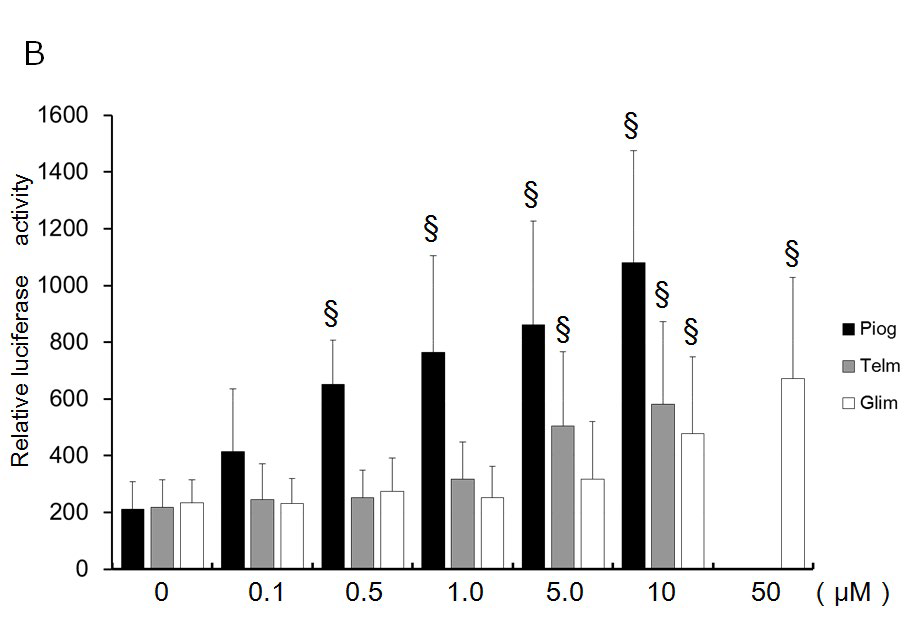
Results are expressed as mean ± SD from ≥4 transfections performed in duplicate. Data were analyzed by ANOVA post hoc tests versus control using Stat View 4.0 software (Abacus Concepts, Berkley, CA, USA). P<0.05 was considered significant. RESULTS Dose-Dependent Transactivation of PPARg by Pioglitazone, Telmisartan, and Glimepiride Telmisartan and glimepiride as well as pioglitazone increased transactivation of PPARg dose dependently using the chimeric receptor GAL4-PPARg and the GAL4-responsive reporter of tk-(MH100)×4-Luc (Figure 1A). Telmisartan ≥1 µM activated PPARg significantly, as did glimepiride ≥5 µM. Transcriptional activity by 10 µM of telmisartan and 10 µM of glimepiride was 58.8% and 49.8% of that by 10 µM of pioglitazone. Telmisartan and glimepiride increased transactivation of PPARg dose dependently on the PPRE using the receptor of PPARg and PPARg responsive reporter (PPRE)×3-Luc (Figure 1B). Transcriptional activity by 10 µM of telmisartan and 10 µM of glimepiride was 53.9% and 49.8% of that by 10 µM of pioglitazone. These results suggest that telmisartan and glimepiride bind to and transactivate PPARγ and thereby act as partial agonists of PPARg.<0.05 was considered significant.
RESULTS
Dose-Dependent Transactivation of PPARg by Pioglitazone, Telmisartan, and Glimepiride
Telmisartan and glimepiride as well as pioglitazone increased transactivation of PPARg dose dependently using the chimeric receptor GAL4-PPARg and the GAL4-responsive reporter of tk-(MH100)×4-Luc (Figure 1A). Telmisartan ≥1 µM activated PPARg significantly, as did glimepiride ≥5 µM. Transcriptional activity by 10 µM of telmisartan and 10 µM of glimepiride was 58.8% and 49.8% of that by 10 µM of pioglitazone. Telmisartan and glimepiride increased transactivation of PPARg dose dependently on the PPRE using the receptor of PPARg and PPARg responsive reporter (PPRE)×3-Luc (Figure 1B). Transcriptional activity by 10 µM of telmisartan and 10 µM of glimepiride was 53.9% and 49.8% of that by 10 µM of pioglitazone. These results suggest that telmisartan and glimepiride bind to and transactivate PPARγ and thereby act as partial agonists of PPARg.
Figure 1: Dose-dependent transactivation of PPARg by pioglitazone, telmisartan, and glimepiride.
(A) Chimeric receptor of GAL4-PPARγ and GAL4 responsive reporter of tkMH100(UAS)×4-Luc were transfected into CV1 cells in the absence or presence of various ligands. Increasing amounts of ligand were added. Luciferase activities induced by pioglitazone (Piog; closed bar), telmisartan (Telm; grey bar), and glimepiride (Glim, open bar) are represented.
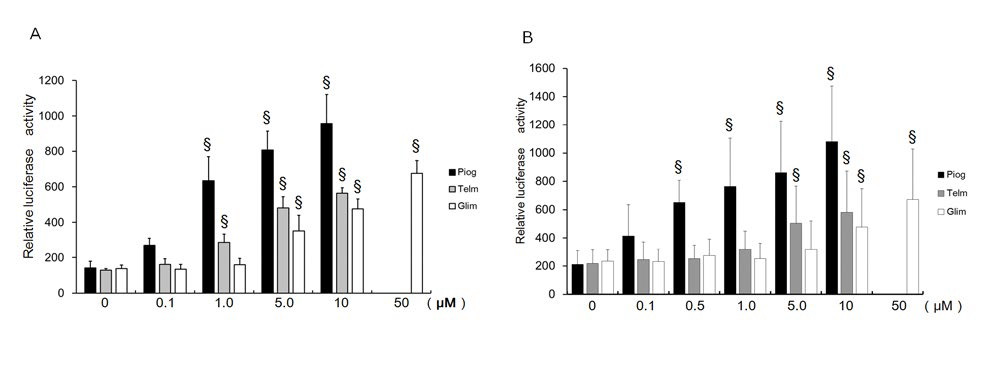
(B) Receptors of PPARg and R×R expression plasmids were transfected into CV1 cells together with PPRE-tk-Luc in the absence or presence of various ligands. Increasing amounts of ligand were added. Luciferase activities induced by Piog (closed bar), Telm (grey bar), and Glim (open bar) are represented. Results are mean ± SD from indicated numbers of transfections in duplicate. *P<0.05; §P<0.05 vs. control (no ligand).
Additive Effects of Telmisartan on Transactivation of PPARg by Glimepiride
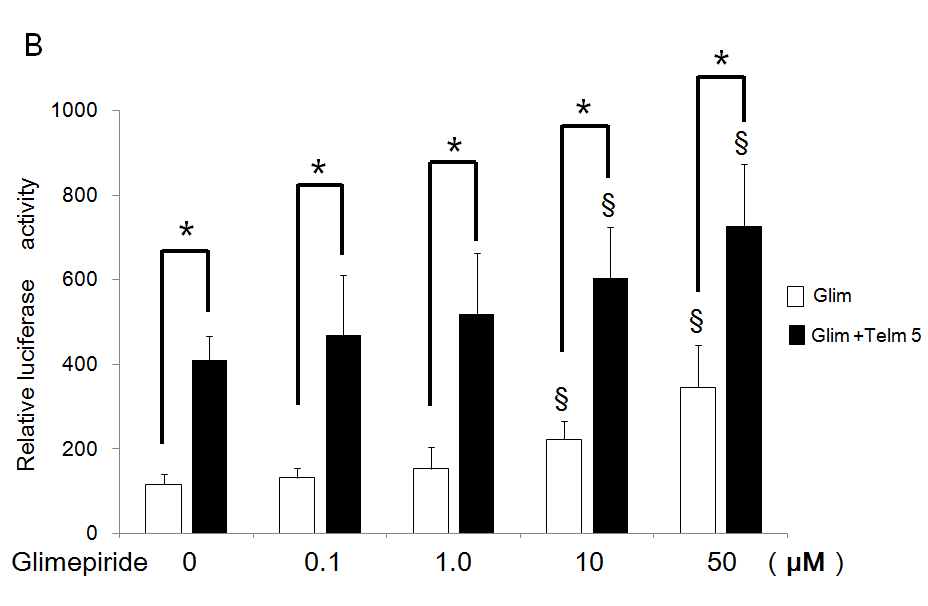
Telmisartan 5 µM potentiated transactivation of PPARg additively by each concentration of glimepiride using the chimeric receptor GAL-4 PPARg and the reporter of tk-(MH100)×4- Luc. Especially, significantly increased activations by 10 and 50 µM of glimepiride were potentiated by addition of 5 µM of telmisartan (Figure 2A). Furthermore, 5 µM of telmisartan potentiated transactivation of PPARg by each concentration of glimepiride on PPRE using the receptor of PPARg and reporter of (PPRE)×3-Luc. In particular, significantly increased activations by 10 and 50 µM of glimepiride were potentiated by addition of 5 µM of telmisartan (Figure 2B).
Figure 2: Additive effects of telmisartan on transactivation of PPARg by glimepiride.
(A) Chimeric receptor of GAL4-PPARg and GAL4 responsive reporter of tk-MH100(UAS)×4-Luc were transfected into CV1 cells in the absence or presence of increasing concentrations of glimepiride together with (closed bar) or without 5 µM of telmisartan (open bar).
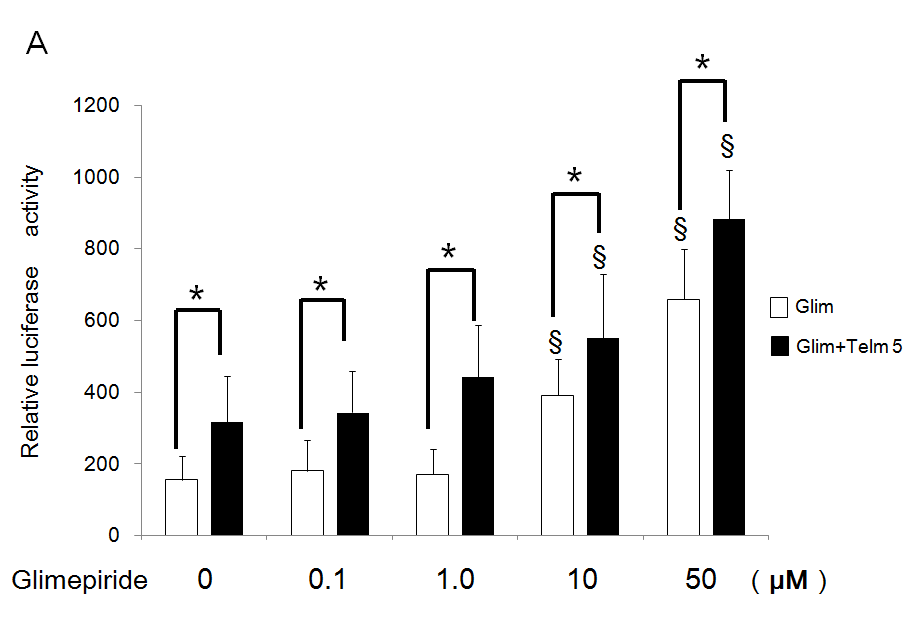
(B) Receptor of PPARg and R×R expression plasmids were transfected into CV1 cells together with PPRE-tk-LUC in the absence or presence of increasing concentrations of glimepiride together with (closed bar) or without 5 µM of telmisartan (open bar). Results are mean ± SD from indicated numbers of transfections in duplicate. §P<0.05vs. respective control: (no ligand).
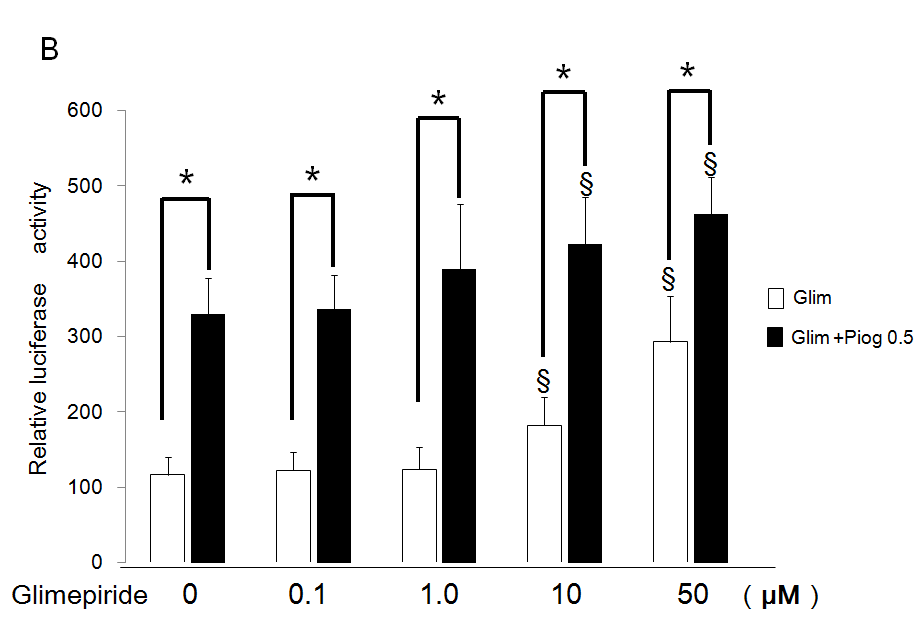
Additive Effects of Pioglitazone on Transactivation of PPARg by Glimepiride
Pioglitazone 0.5 µM potentiated transactivation of PPARg additively by each concentration of glimepiride using the chimeric receptor GAL4-PPARg and the reporter of tk- (MH100)×4-Luc. Especially, significantly increased activations by 10 and 50 µM of glimepiride were potentiated by addition of 0.5 µM of pioglitazone (Figure 3A). Furthermore, 0.5 µM of pioglitazone potentiated transactivation of PPARg by each concentration of glimepiride on PPRE using the receptor of PPARg and reporter of (PPRE)×3-Luc. In particular, significantly increased activations by 10 and 50 µM of glimepiride were potentiated by addition of 0.5 µM of pioglitazone (Figure 3B).
Figure 3: Additive effects of low concentration of pioglitazone on transactivation of PPARg by glimepiride.
(A) Chimeric receptor of GAL4-PPARg and GAL4 responsive reporter of tkMH100 (UAS)×4-Luc were transfected into CV1 cells in the absence or presence of increasing concentrations of glimepiride together with (closed bar) or without 0.5 µM of pioglitazone (open bar).
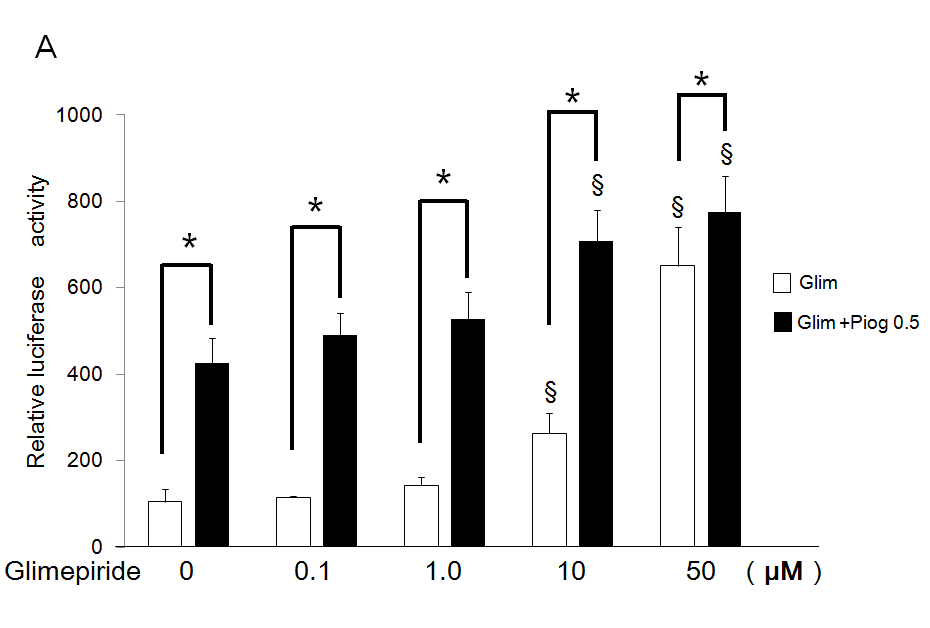
(B) Receptor of PPARg and R×R expression plasmids were transfected into CV1 cells together with PPRE-tk-Luc in the absence or presence of increasing concentration of glimepiride together with (closed bar) or without 0.5 µM of pioglitazone (open bar). Results are mean ± SD from indicated numbers of transfections in duplicate. *P<0.05; §P<0.05 vs. control (no ligand).
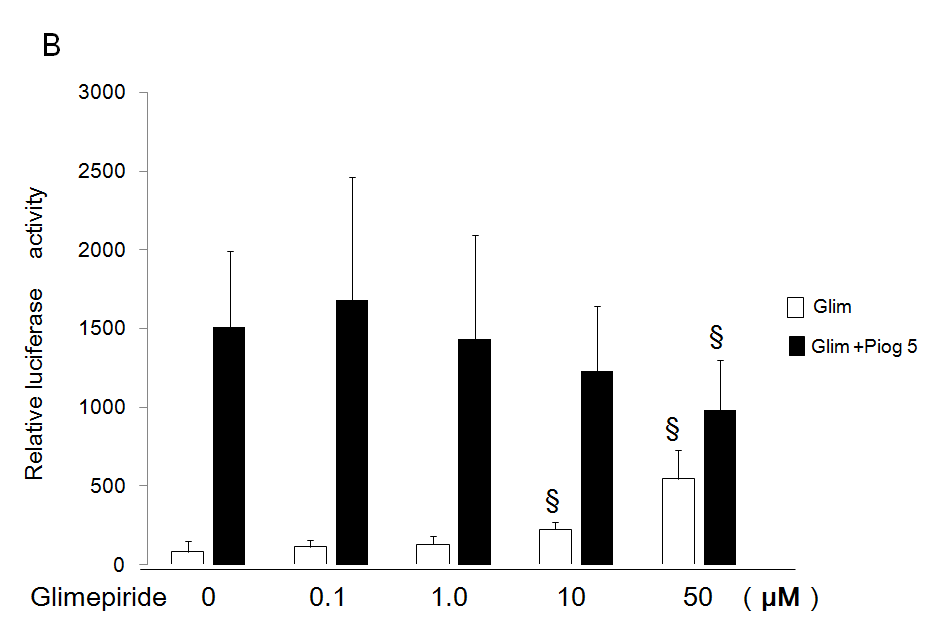
Antagonistic Effects Glimepiride on Transactivation of PPARg by Pioglitazone
High concentrations (10 and 50 µM) of glimepiride reduced transactivation of PPARg by 5 µM of pioglitazone using the chimeric receptor GAL4-PPARγ and the reporter of tk- (MH100)×4-Luc (Figure 4A). Moreover, 50 µM of glimepiride reduced transactivation of PPARg by 5 µM of pioglitazone using the receptor of PPARg and reporter of (PPRE)×3-Luc (Figure 4B).
Figure 4: Antagonistic effects of glimepiride on transactivation of PPARg by pioglitazone.
(A) Chimeric receptor of GAL4-PPARg and GAL4 responsive reporter of tk-MH100(UAS)×4-Luc were transfected into CV1 cells in the absence or presence of increasing concentration of glimepiride together with (closed bar) or without 5 µM of pioglitazone (open bar).
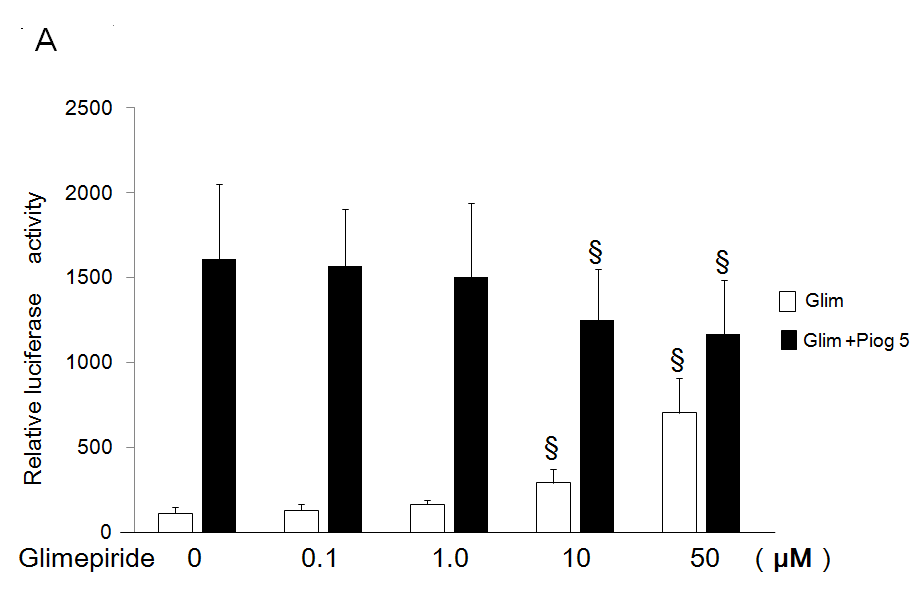
(B) Receptor of PPARg and R×R expression plasmids were transfected into CV1 cells together with PPRE-tk-LUC in the absence or presence of increasing concentration of glimepiride together with (closed bar) or without 5 µM of pioglitazone (open bar). Results are mean ± SD from indicated numbers of transfections in duplicate. *P<0.05; §P<0.05 vs. control (no ligand).
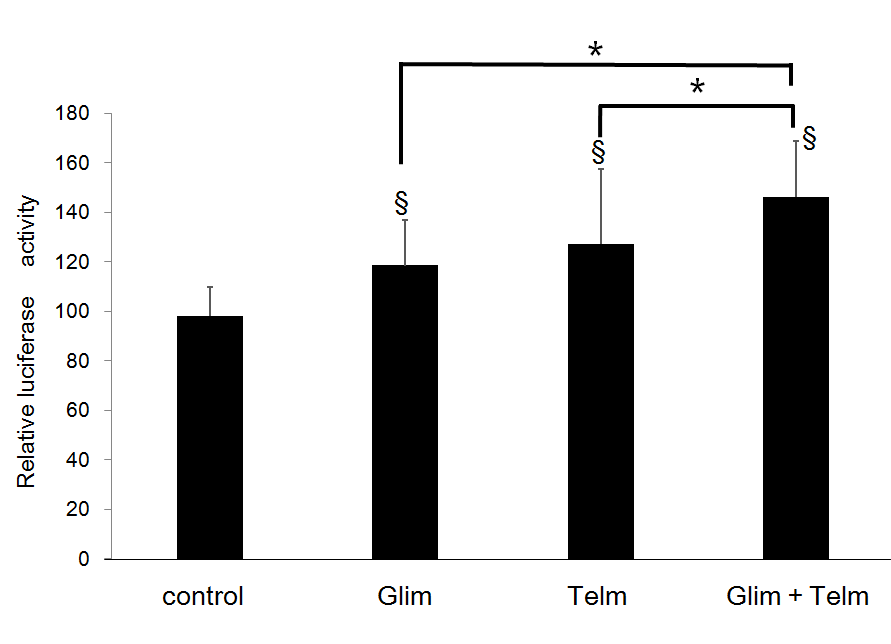
Additive Binding Activity of PPARg to SRC-1 by Glimepiride and Telmisartan
To examine the additive effects of glimepiride and telmisartan on the interaction of PPARg with co-activator, mammalian two-hybrid assay using VP16-PPARg, GAL4-SRC-1, and the reporter of GAL-Luc was performed. Both glimepiride and telmisartan enhanced Luc activity by the binding between VP16-PPARg and GAL4-SRC-1. The interaction between VP16- PPARg and GAL4-SRC-1 was more enhanced by addition of both glimepiride and telmisartan than by that of glimepiride or telmisartan alone (Figure 5).
Figure 5: Additive effects of glimepiride and telmisartan on binding between PPARg and SRC-1.Receptor of GAL4-PPARg and SRC-1 expression plasmids were transfected into CV1 cells together with tk-MH100(UAS)×4-Luc in the absence or presence of glimepiride and/or 5 uM of telmisartan. Results are mean ± SD from indicated numbers of transfections in duplicate. *P<0.05; §P<0.05 vs. control (no ligand).
DISCUSSION
Telmisartan directly activated PPARg dose dependently; telmisartan 10 µM activated transcriptional system using GAL4-PPARg chimera receptor and GAL4 responsive reporter gene and that using receptor of PPARg and reporter gene including PPRE to 58.8% and 53.8% of the level achieved by 10 µM of pioglitazone, respectively. These data suggest that telmisartan binds and activates PPARg because the structure of telmisartan is similar to that of TZD. Glimepiride 10 µM also activated PPARg to 49.8% of the level by 10 µM of pioglitazone in similar conditions. Although the structure of glimepiride is different from that of TZD, these data suggest that nonetheless glimepiride binds and activates PPARg. The binding pocket of PPARg is large and glimepiride might bind to PPARg in different manner from TZD.
These two ligands act as partial agonists of PPARg as previously reported, although other ARBs (except irbesartan), and other sulfonilureas do not increase transactivation of PPARg.6,7,8,22 CV1 cells were used in the present experiment to estimate the interactions of these ligands. It would be necessary to confirm these interactions in adipocytes expressed of PPARg. Telmisartan binds to helixes H3 and H7 of LBD of PPARg does TZD and other partial agonists of PPARg.7,26,27 Difference of binding ability (to H12) might bring different degrees of activation of PPARg. The carboxy terminal activation domain (AF-2) in H12 of LBD is proposed to undergo induced conformational changes after binding to ligand.20,21,28,29 The present experiment showed that two ligands, glimepiride and telmisartan, had additive activations of PPARg, suggesting that each ligand did not change binding capacity to the receptor of PPARg for each ligand. Recent study showed that some combinations of ligands, fatty acid and indole acetate or indomethacin, induce additive orsynergistic activation of PPARg.27 This study confirmed the data about the combination effect of telmisartan and glimepiride. We do not have definitive data about the binding site of glimepiride to PPARg. However, it was reported that glimepiride competitively binds PPARg with rosiglitazone, suggesting that glimepiride has partly similar binding site of PPARg to TZD.18
Because different ligands of PPARg might recruit coactivator in different manner, interactions about the recruitment of coactivator by each ligand should be investigated. The present results suggest that telmisartan, glimepiride, and pioglitazone induced interactions between PPARg and SRC-1 depending on the transcriptional activity of PPARg (data not shown). Glimepiride also induced additive interaction between PPARg and SRC-1. As for SRC-1, these ligands recruited the coactivator to PPARg depending on the activity, although different ligands were reported to recruit coactivators such as DRIP205, TIF-2, and PGC-1 in different manners.6,8,20 A previous article reported that DRIP205 was recruited by rosiglitazone and telmisartan in similar degree, and PGC-1 by rosiglitazone and telmisartan depending on the transcriptional activity of PPARg, whereas TIF-2 was recruited not by telmisartan but by rosiglitazone. These findings suggest that SRC-1 and PGC-1 were recruited by ligands depending on the transcriptional activity of PPARg, but not TIF-2. Different manners of cofactor recruitment would result in different induction of genes. Schuup, et al.6 suggested the genes of prostacyclin receptor and glycerol kinase induced by TZDs were less stimulated by ARBs, and that these ligands had some transcriptional activity of PPARg with less side effects including edema or body weight gain.
A number of clinical trials reported that telmisartan improved insulin sensitivity and lowered plasma triacylglycerides in type 2 diabetic patients.30,31,32 These effects were remarkably prominent by administration of telmisartan among all ARBs, suggesting that the observed effects might originate from activation of PPARg. Glimepiride has been reported to stimulate insulin secretion with less increasing of body weight. These effects might originate from stimulating activity of PPARg with less effect inducing edema or retention of body fluid. Clinically, combination therapy of telmisartan with glimepiride is suggested to induce additive effect on activity of PPARg. Although clinically approved doses of telmisartan and glimepiride individually have only slight effect on activation of PPARg, the combination therapy of both drugs might bring additive effects on activation of PPARg. Although we have no definite data about combination therapy of glimepiride and telmisartan improving insulin sensitivity, these drugs would potentially induce additively transcriptional activity of PPARg without side effects. This combination therapy might provide a new therapeutic option for better cardiovascular management in metabolic diseases and might initiate the development of new classes of combination therapy. Furthermore, activity of PPARg by natural ligand might be potentiated by these agents.
On the other hand, our data showed that high concentrations of glimepiride and telmisartan inhibited activity of PPARg by pioglitazone. Previous in vitro experiments showed that partial agonists inhibited adipocyte differentiation stimulated by TZDs.33 However, it seems improbable that glimepiride and telmisartan would clinically inhibit activity of pioglitazone on PPRE, because clinical doses of glimepiride and telmisartan bring only low concentration (1 µM) in blood stream. Contrarily, there are some reports that telmisartan had some beneficial metabolic effects in type 2 diabetic patients and animal models treated with rosiglitazone. Derosa, et al.34 reported that administration of telmisartan improved glucose tolerance in type 2 diabetic patients treated with rosiglitazone without body weight gain. Zanchi, et al.35 reported that telmisartan prevented pioglitazone-induced weight gain in Zucker fatty rats without interfering with its insulin-sensitizing properties. It has been suggested that telmisartan prevents weight gain and obesity through activation of PPAR delta-dependent pathways.36 Clinically, combined administration of telmisartan and low-dose pioglitazone would be an attractive therapy with respect to weight control because it might improve insulin sensitivity with less increase of edema than pioglitazone monotherapy.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
ACKNOWLEDGMENTS
We thank Dr. Makishima and colleagues at Nihon University for plasmids used in this study.














