INTRODUCTION
Acute Lymphoblastic Leukemia (ALL) peak age 2 to 5 years is the most common (30%) form of all childhood malignancies.1 Central Nervous System (CNS) complications are well documented in ALL. Acute polyneuropathy before, during, and after the chemotherapy has been documented in adults and children. However, it is a rare complication of ALL. It is usually induced by vincristine or Guillain-Barre Syndrome (GBS). The clinical manifestation, Cerebrospinal fluid (CSF), and neurophysiologic studies are the cornerstones in making the diagnosis of acute polyneuropathy.2 The chemotherapeutic drugs, vincristine, methotrexate and cytarabine, and CNS radiation are essential components of ALL chemotherapy.
We report a 5-year-old boy with ALL who developed acute symmetrical polyneuropathy. Based on a systemic review of the literature, we provide and discuss the neurologic profile and differential diagnosis of the acute polyneuropathy in childhood leukemia.
CASE REPORT
A 5-year-old newly diagnosed pre-B-Cell acute Lymphoblastic Leukemia (ALL) boy was on the Children Oncology Group (COG) ALL 0932 chemotherapy protocol. This consists of intrathecal cytarabine 70 mg on day 1, vincristine 1.2 mg intravenous push over a minute on day 1, 8, 15 and 22, pegaspargase 1950 IU intravenous infusion over an hour on day 4, and intrathecal methotrexate 12 mg on day 8 and 29. Prior to admission, he has had completed the
first course of induction chemotherapy except for the final dose of methotrexate. He received no CNS radiation.
He presented with bilateral leg pain for the past 4 days. He was unable to bear his weight or walk. He had no trauma, antecedent infection, or family history of hereditary neuropathy. Home medications beside chemotherapy included acetaminophen with codeine, fluconazole, bactrim, ondansetron, lansoprazole, and dexamethasone. The pain responded to intravenous morphine. On the 20th day of induction therapy, he developed episodes of painful erections and micturition difficulty. Urological assessment ruled out any anatomic concerns. His urinalysis and urine culture, both were normal. He was evaluated by hematology and rehabilitation medicine.
On examination he was comfortably lying in the bed. His vitals and temperature were normal. His weight was 24.4 kg (90th percentile) and height was 119.6 cm. (97th percentile). Liver was palpable 4 inches below the right subcostal margin. Neurological examination revealed normal cranial nerves. His legs were flaccid and he had no strength as compare with 3/5 muscle power in his arms. The leg movements elicited pain. Deep tendon reflexes were decreased in both legs. Cold sensation, joint position, and vibration were normal. He had no muscle tenderness or fasciculation.
Laboratory investigations revealed normal creatine phosphokinase and aldolase. Contrast-enhanced T1-weighted sagittal and axial Magnetic Resonance Images (MRI) of the lumbosacral spinal revealed enhancement of the anterior (ventral) roots (Figure 1(A) and (B). CSF study revealed 18 wbc/ µL (0-5), 2 rbc/ uL (0), no blast cells, protein 160 mg/dL (15-45), glucose 53 mg/dL (50-80), myelin basic protein 2.19 ng /ml (0.00-1.10). Nerve conduction studies indicated motor ax onopahty in the several examined nerves with no evidence of demyelination.
Figure 1 (A) and (B): Contrast enhanced T1-weighted magnetic resonance sagittal (A) and axial (B) images of the lumbosacral spine show enhancement of the anterior (motor) roots in the distal spinal cord (arrows). Please note there is no enhancement of the posterior roots.
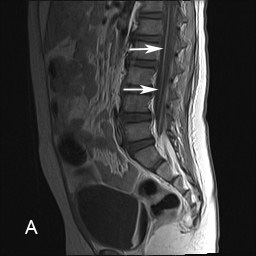
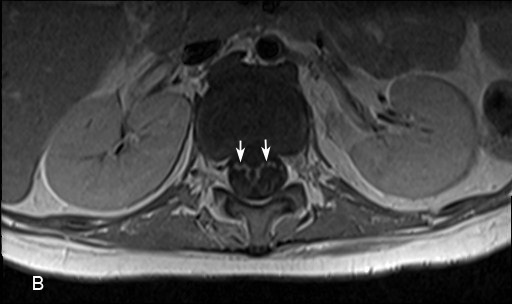
Foot Note: Although enhancement of the cauda equina is a non-specific neuroimaging findings finding but its presence in appropriate context suggests polyradiculopathy. This provides insight for an etiologic evaluation.
Upon his mother’s request, he was transferred to the University of Michigan Medical Center where he developed swallowing difficulty requiring nasogastric feeding and spastic neurogenic bladder requiring urinary catheterization. He received Intravenous Immunoglobulin (IVIG) in a dosage of 0.4 gm/kg/day for 5 days. Once his neurological status plateaued, he resumed a modified COG chemotherapy Protocol ALL 1131 in which vincristine and methotrexate were withheld. After three months, a follow-up MRI of the lumbosacral spine revealed decreased enhancement of the anterior nerve roots. After four months of presentation, he had no functional motor improvement and he needed a wheel chair for ambulation.
DISCUSSION
Table summarizes the results of the systemic review of the literature of neurologic manifestations in children with acute lymphocytic leukemia.3,4,5,6,7,8
| Authors [Reference]Year |
Age, Sex |
Onset and Neurologic Features in relation to Chemotherapy |
Spinal MRI1, Elevated CSF2 protein/
CSFWBC3 |
Authors’ Diagnosis |
Interventio
IVIG4 given/ Drug discontinued |
Outcome
|
| Norman M et al3 1987 |
3.6 year, Male |
Day 19th
Ascending paresthesia and flaccid quadriparesis, and respiratory failure |
Not available/Yes, No
|
GBS5 potentiating the vincristine toxicity |
No/Vincristine |
A slow recovery in the arms after 18 months |
| Aral YZ et al4 2001 |
4 year, Male |
Month 3rd
Difficulty walking , leg pain, weakness, and the left facial nerve palsy |
Not available/Yes, No |
GBS |
Yes/NA |
A complete recovery in six weeks |
| Anderson SC et al5 2002 |
3 year,
Female |
Day 15th
Progressive quadriparesis and areflexia over 2 weeks period, received no vincristine |
Yes/Yes, No
|
Methotrexate intrathecal induced ventral
polyradiculopathy |
Yes/Intrathecal methotrexate |
Complete recovery in four weeks |
|
Ray M
et al6 2002 |
3 year, Male |
Day 14th
Flaccid quadriparesis, difficulty micturition, loss of vision, downward gaze palsy, loss of gag reflex, and respiratory failure |
Not available/
Not available,
Not available
|
Vincristine
toxicity |
No/No
|
Died on 33 rd. day of Induction therapy |
| 7 year, Male |
Day 24th
Flaccid quadriparesis, III, IV,, IX, X cranial nerves paresis, generalized seizure, and respiratory failure |
Not available/
No, No |
Vincristine
toxicity |
No/No
|
Died on the 32nd day of induction chemotherapy |
| Yun Woo
Chang et al7 2003 |
4.3 year, Female |
Year 2
Progressive and ascending extremities weakness, No sensory symptoms |
Yes/No, No |
Vincristine induced motor
paraparesis |
No/Vincristine |
Assisted walking at age 9 months of follow up |
|
Rajeshwari
B. et al8
2013 |
6 year,
Male |
Week 5
Progressive symmetrical limbs weakness and facial nerve palsy, No sensory symptoms |
Not available/
Yes, Yes |
Acute motor axonal neuropathy a variant of GBS |
Yes /No |
8 weeks later, a full recovery to normal. |
| 2 year,
Male |
Week 4
Rapidly progressive ascending paralysis and respiratory failure
|
Not available/No, No |
Acute motor axonal neuropathy variant of GBS |
Yes/No |
Initial improvement during IVIG therapy, but succumbed to sepsis. |
| Current
Study
2014 |
5 year,
Male |
Day 19th
Leg pain, quadriparesis, difficulty micturition , priapism, and difficulty swallowing |
Yes/Yes, Yes |
GBS/ALL potentiating the vincristine toxicity |
Yes/Vincristine and Methotrexate |
In wheel chair after 4 months after therapy |
Foot Note: The diagnosis of acute neuropathy in ALL is based on the neurologic manifestations, spinal MRI, the typical CSF findings, and decreased conduction velocities in the affected peripheral nerves. GBS and vincristine induced polyneuropathy are rare but these are two common type of axonal neuropathy in children with ALL.
1MRI: Magnetic Resonance Image; 2CSF: Cerebrospinal Fluid; 3WBC: White Blood Cell counts; 4IVIG: Intravenous Immunoglobulin; 5GBS: Guillain-Barre Syndrome |
Since 1987 to 2014 a total of 9 children with ALL aged 3-7 years developed neurologic symptoms. The majority (7/9) were boys. Onset of the symptoms in relation to chemotherapy varied from 14 days to 2 years. The most common neurologic presentation was refusal or inability to walk. Five children were diagnosed with GBS, 3 children with vincristine induced polyneuropathy, and one child had methotrexate induced polyneuropathy. The cranial nerve/s involvement with or without respiratory failure were common (7/9). Most commonly affected cranial nerve in both systemic and meningeal ALL optic nerve was conspicuously absent from these reports.9 The seizure was uncommon (1/9).
Why Axonal Neurotoxicity?
Vincristine inhibits microtubule formation resulting into an arrest of dividing cells. This commonly produces an asymptomatic axonal rather than a demyelinating polyneuropathy.10 GBS in children with no ALL commonly causes demyelinating polyneuropathy. The common finding of axonal injury in children with ALL leukemia suggests vulnerability of the axons in ALL. Studies suggest that individual susceptibility and younger age contribute to the likelihood of neurotoxicity. Vincristine neurotoxicity relates to the plasma peak concentration rather than to the total dosage.11 Our patient had no evidence or family history of a pre-existing hereditary neuropathy like Charcot-Marie-Tooth disease. But the children with ALL along with pre-existing hereditary neuropathy have an increased risk for vincristine axonal neurotoxicity.12 Vindesine like vincristine a vinca alkaloid which is used as an alternative for vincristine causes a similar neurotoxicity.13
DIAGNOSIS
Given the complexity of children concurrently receiving multiple neurotoxic drugs and presenting with variable neurologic presentations, the question arises whether emergence of a new symptomology is the result of ALL, ALL relapse, neurotoxicity of the drugs in use or a new onset second condition like GBS.
The initial presentation of acute polyneuropathy particularly GBS is vague. At times, an erroneous diagnosis of viral or cerebellar dysfunction is made. Because these complexities likely to interrupt ongoing chemotherapy or hinder initiation of IVIG therapy, prompt differentiation of these medical and neurologic entities are of practical importance.
Differential Diagnosis
Bone marrow infiltration causing bony tenderness or pain and pathological fractures can be early manifestations of ALL14 Infarction or hemorrhage into the nerves and leptomeninges caused by direct leukemic cell infiltration are uncommon. Nonetheless, it has been reported as the presenting neurologic manifestation of ALL. It usually develops during the induction chemotherapy. But a symmetrical polyradiculopathy presenting 6 months before the onset of ALL has been described.15 A focal or an asymmetric motor or sensory symptoms affecting limb/s is possible. Cranial nerves III, IV, and VI involvement either in isolation or combination can be presenting manifestations of ALL.16
Refusal to bear weight or inability to walk due to pain or weakness in an apparently healthy child with no history of trauma should raise the possibility of neurologic etiology. In fact an inability to walk in a child is the most common presentation of an acute polyneuropathy or cauda equina syndrome. ALL, GBS, vincristine, methotrexate, and cytarabine administration, all are known to cause acute polyneuropathy.17 Acquired polyneuropathy is characterized by an asymmetric involvement. But polyneuropathy caused by GBS or vincristine neurotoxicity despite being an acquired polyneuropathy is usually bilateral symmetrical.18 Autonomic dysfunctions in the form of gastrointestinal or urinary system disturbance, impotence, priapism, and orthostatic hypotension can also occur. Its involvement with or without painful lower limbs occurs in the cauda equina syndrome and should prompt spinal cord compromise. Acute CNS complications, seizures and acute encephalopathy are extremely rare, but have been reported.19
Methotrexate, cytarabine, and corticosteroids (triple-drug therapy) are the most common drugs used intrathe- -cally. They produce both central and peripheral nervous systems toxicity. The most common presentation of methotrexate toxicity is an acute encephalopathy, stroke-and seizure-like activity, spinal cord disturbance, and cauda equina syndrome.20,21 Cytarabine can affect any part of the nervous system but cerebellar syndromes, myelopathy, and peripheral neuropathy occur in a decreasing frequency.
It should be noted that although we did not perform test for porphyria to entertain the differential diagnosis of acute axonal polyneuropathy with autonomic nerve system dysfunction or rule out rarely occurring acute form of chronic inflammatory demyelinating polyneuropathy, which may mimic the clinical picture of classical GBS. However, both of these conditions should be considered.
Spinal Magnetic Resonance Imaging
Spinal MRI with and without contrast confirms the clinical impression of spinal cord compression, vertebral collapse, epidural leukemic mass, and transverse myelitis. It is also useful in demonstrating cauda equina, anterior or posterior roots, contrast enhancement which suggests a break in the blood-brain barrier.22 Although enhancement of the cauda equina is a non-specific finding but its presence in appropriate clinical context suggests polyradiculopathy. This provides insight for an etiologic evaluation. Enhancement can also be caused by neurotoxicity after an intrathecal administration of methotrexate. It should be noted that an early MRI can be normal in patients with acute myeloencephalopathy. The literature review in the assessment of peripheral neuropathy suggests infrequent (3/9) use of spinal MRI. But it is probably the single most important initial investigation in those children in whom the cause of the gait difficulty remains unclear.
Diagnostic Challenge
Differentiating GBS from vincristine-induced toxicity in children with ALL is a diagnostic challenge. Both conditions affect the peripheral nervous system and have similar CSF, spinal MRI, and neurophysiologic findings. But vincristine toxicity might progress to involve the CNS. The absence of a clear infectious trigger, negative bacterial and viral studies, and the absence of anti-ganglioside antibodies makes vincristine toxicity more likely.
Treatment and Outcome
The children with diagnosis of GBS received empirical IVIG except the earliest reports of Norman M et al 1987. Interrupting chemotherapy will usually reverse the acute polyneuropathy. But if the neuropathy is caused by ALL or its relapse, chemotherapy may need to be intensified. Plasmapheresis and IVIG therapy are indicated in GBS. Both have similar efficacy, but for practical reasons IVIG is the preferred initial therapy.23 The use of IVIG in chemotherapy-induced neuropathy is empirical.
Depending upon the cause and severity, the neurologic outcome has been variable, ranging from a full recovery to death (Table 1). It should be noted that 4/9 children with ALL developed respiratory failure. The children with respiratory failure had highest number of deaths; three out of 4 children. Our patient developed severe motor and sensory axonal neuropathy, neurogenic bladder, priapism, and difficulty swallowing evolving over several weeks. We believe that he had GBS possibly induced by the neurotoxic effect of vincristine or an altered immunologic status secondary to ALL. However, he did not develop respiratory failure or sepsis.
CONCLUSION
Reported cases in the literature suggest that other than areflexia acute polyneuropathy in children with ALL receiving chemotherapy is rare. A progressive ascending sensory and motor sign and symptom are likely due to GBS or vincristine-induced neurotoxicity. Lumbosacral MRI showing root enhancement, CSF studies, and an abnormal nerve conduction study facilitate early diagnosis. Early recognition of acute polyneuropathy will facilitate a judicious use of immunotherapy for GBS, very much needed lifesaving chemotherapy, and prevent a high mortality and morbidity in children with acute childhood leukemia.
ACKNOWLEDGMENTS
We thank J. Melrose a Medical Student for her assistance for organization of the history of the case and Dawn Fields a neurologic nurse practitioner, and Samira Naime, MD for reviewing this manuscript.
AUTHORS’ CONTRIBUTION
Vikash S. Gupta reviewed the literature, prepared an initial version of the draft, and has approved the final version of this manuscript.
DECLARATION OF CONFLICTING INTEREST
The authors have no conflicting interest, commercial, or other financial relationships related to this article.







