BACKGROUND
Transfusion of packed red blood cells (PRBCs) is indicated in severely bleeding patients and in anemic patients who require increased oxygen-carrying capacity. According to the 2016 AABB guidelines, for patients who are hemodynamically stable without active bleeding, PRBC transfusion is likely indicated for hemoglobin of 6 to 7 g/dL.1 A higher threshold (<8 g/dL) is indicated in patients with cardiovascular disorders.
Transfusion of PRBCs is associated with an increased risk of adverse reactions. Complications may occur over a wide range of time from immediately during transfusion to days afterwards. These include allergic reactions, febrile non-hemolytic reactions, hemolysis, transfusion associated circulatory overload (TACO), transfusion-related acute lung injury (TRALI), transfusion-transmitted pathogens, and immune-mediated reactions. Transfusion-associated sepsis (TAS) is an acute nonimmune reaction that is more common with platelet transfusion at an incidence of 1:25,000 for pooled platelets and 1:50,000 for single donor platelets. The estimated incidence of TAS in PRBC transfusion is 1:250,000.2 Due to low storage temperatures of PRBCs, the most significant hazards are psychrophilic or cryophilic organisms that grow in cold (4 °C) temperatures and use citrate as a nutrient such as Pseudomonas species, Yersinia enterocolitica, Campylobacter, and Serratia species.3 Contamination of PRBC units most likely occurs due to non-aseptic technique during phlebotomy, contaminated equipment used for blood collection and processing, and rarely due to its presence in the donor blood at the time of donation.
CASE
A 29-year-old woman with microangiopathic hemolytic anemia associated with Systemic Lupus Erythematosus (SLE) and Lupus nephritis was admitted to the hospital for lupus flare. Her medical history indicated that she received Rituximab and Cytoxan infusion during her previous admission and had been receiving Cytoxan infusions as an outpatient. Her current hospital course was complicated by thrombotic thrombocytopenic purpura (TTP), hemolytic-uremic syndrome (HUS), renal failure, and pericardial effusion with tamponade. Plasmapheresis procedures were initiated twice a week concurrent with hemodialysis three times a week as an outpatient.
A few weeks later, the patient came to the apheresis unit for her regular out-patient plasma exchange procedure. She felt well and did not report any pain, fever, chills, or shortness of breath. Her pre-procedure vitals were stable (blood pressure (BP) 138/75 mmHg, hematocrit (HCT) 23, platelet count (PLT) 110). Her only complaint was fatigue which was thought to be secondary to hypokalemia (K+ was 2.8 mEq/L two days earlier and was currently 3.3; normal range 3.2-5.5 mEq/L). She also complained of loose stools after taking labetalol, which was a common side effect for her. However, this may have been due to Costridium difficile as she tested positively on admission to the ICU. She took all of her medications earlier that morning except labetalol (800 mg twice/day with instructions to hold for systolic blood pressure (SBP)<113 mmHg) because her SBP at home was 106 mmHg. The apheresis procedure started at 8:40 am for 1x volume plasma exchange. The patient was pre-medicated with Tylenol (650 mg PO) and hydrocortisone (100 mg IV) and tolerated apheresis well. The patient received 11 units of plasma during the apheresis procedure, which was concluded successfully at 10:30 am. Due to a chief complaint of fatigue and a HCT of 23, one unit of PRBCs was ordered by her hematologist to be transfused after the apheresis procedure.
Her current type and screen test (TST) result was O-Negative (consistent with her medical record) with a positive antibody screen identified as Anti-D due to Rho Immune Globulin. A full-crossmatched, O Negative PRBC was released for transfusion at 10:42 am. The PRBC transfusion was started at 10:52 am at a rate of 100 mL/hr. The patient’s pre-transfusion vitals were: temperature=37 °C, BP 138/75, and pulse 97. At 12 pm the patient experienced a sudden sharp occipital headache that she rated as 7 (on a scale of 1-10), back pain, heaviness of her lower extremities, and reported “I cannot get comfortable”. The transfusion was stopped. BP was found to be slightly elevated at 154/94 mmHg. She received labetalol (800 mg PO), Lasix (20 mg IV), oxycodone (5 mg PO), Tylenol (650 mg PO), and hydrocortisone (100 mg IV). Twenty minutes later the patient felt better and her BP was 159/96 mmHg; another dose of Lasix (20 mg IV) was administered. Following a consultation with one of the hematology fellows, the apheresis nurse was advised that this was most likely an allergic reaction and the nurse was informed that she could restart the PRBC transfusion. As the patient was feeling better, the same PRBC transfusion was restarted 1.5 hours later at a rate of 100 mL/hr. BP then was 104/54 mmHg, heart rate 117 bpm, and temperature 37.6 °C. Within 15 minutes of restarting the transfusion, the patient became pale and her BP dropped further to 98/47 mmHg with a heart rate of 115 bpm. The patient expressed concern about her blood pressure so to overcome this slight hypotension the transfusion rate was increased to 150 mL/hr. Five minutes later she was reported to have rigors, was crying and reported being very scared and emotional, and complaining of crushing chest pain and shortness of breath. At this time, BP was found to be 87/40 mmHg, heart rate in the 50s, respiration in the 40s, temperature of 37.3 °C, and O2 saturation low at 60% on room air and 80% on 100% oxygen non-rebreather mask. The PRBC transfusion was discontinued after a total of 150 mL was administered, and the patient received Benadryl (50 mg IV), hydrocortisone (100 mg IV), and Demerol (25 mg IV) and was sent to the emergency department (ED) for the crushing chest pain. Upon arrival in the ED the patient felt warm to the touch and had a temperature of 38.6 °C. One of the patient care teams evaluating the patient felt the reaction was acute and was likely transfusion-related acute lung injury (TRALI). The remaining PRBCs and tubing were sent to the blood bank to complete a transfusion reaction work-up. A fresh patient’s blood sample was also sent to the microbiology laboratory for a sterility blood culture.
A Transfusion Reaction workup was initiated immediately at the blood bank. Clerical errors were ruled out. No hemolysis in the returned PRBC unit was obvious (19-days-old). However, moderate hemolysis in the patient’s post-reaction specimen was observed. The post-reaction patient testing was confirmed to be O Negative with a negative direct antiglobulin test (DAT). Repeat ABO testing of the PRBC unit matched the collection center label. Repeat testing of the patient’s pre-reaction specimen agreed with the original results. Repeat testing of the full-crossmatch to both the patient’s pre-reaction specimen and post-reaction specimen confirmed compatibility of the unit.
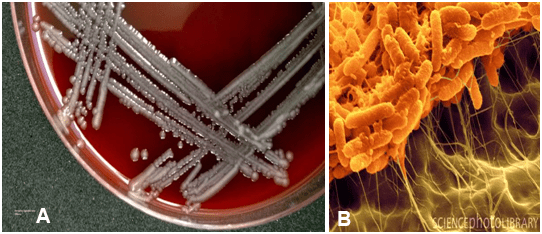
It was noted by one of the blood bank staff that the PRBC looked darker in color (Figure 1A, 1B and 1C). This color became progressively darker the longer the unit was exposed to room temperature. Several of the segments attached to the bag were also darker and several (those farthest from the bag) remained bright red with a normal appearance. Due to the moderate hemolysis in the patient’s post-reaction specimen, the blood bank interpreted the transfusion reaction as a possible acute hemolytic reaction with no further transfusions allowed at the time. The product bag and all segments were sent to the Microbiology laboratory for gram stain and sterility blood cultures. Meanwhile, the patient’s temperature was reported to be up to 38.6 °C. Two hours later the gram stain of the PRBC bag was reported positive for gram variable bacilli. Three days later the sterility blood culture indicated Serratia liquefaciens with colony forming units/mL too numerous to count (Figure 2A and 2B). The noticeably darker segments attached to the unit were also found to contain Serratia liquefaciens while the segments that remained normal in appearance had no growth. The same bacteria were retrieved in the patient’s post-transfusion blood sample. The interpretation of the transfusion reaction was modified to bacterial sepsis caused by contamination of the PRBC unit.
Figure 1: A) Represents a Dark Red Color of Contaminated PRBC (Actual Photo of Contaminated PRBC); B) Represents the Bright Red Color of Normal ~20 Days Stored PRBC; C) Segments Attached to the Contaminated Unit, some being Dark and some being Normal in Color.
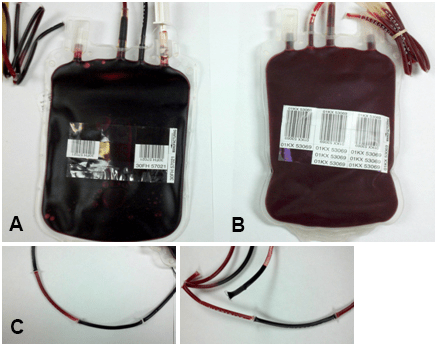
Figure 2: A) Serratia liquefaciens on Blood Agar. August 2014. www.microregistrar.com. May 2017 B) Serratia liquafaciens Biofilm SEM. Scimat/Science Photo Library. May 2012. microbewiki.kenyon.edu. May 2017
While in the ED, the patient received Solumedrol (125 mg IV) and Lasix (20 mg IV). Her chest X-ray showed moderate pulmonary edema along with worsening pleural effusion. She was then admitted to the medical ICU for septic shock management, which included broad spectrum antibiotic therapy and inotropic support with norepinephrine bitartate (Levophed, Phizer, New York, New York, USA) infusion. Infectious disease was consulted and she was treated with ceftriaxone (2 g IV/day for 17 days) and tobramycin (300 mg IV/day for 6 days). She was also treated for Clostridium difficile, which she likely was infected with prior to admission, and completed a course of metronidazole (500 mg IV/8 hours for 23 days) and vancomycin (1,000 mg IV for 2 days and 125 mg PO/6 hours for 3 doses). Antibiotic treatment was effective as evidenced by improvement in leukocytosis per the infectious disease service.
At the time of admission to the ICU the patient displayed signs of cardiac tamponade due to a worsening chronic effusion. A drain was placed and 800 mL of fluid was removed with another 250 mL drained in 24 hours. After the procedure the norepinephrine bitartrate infusion was weaned and the patient was transitioned to a vasopressin (Vasopin, Samarth Pharma Pvt. Ltd., Mumbai, Maharahtra, India) infusion for 24 hours. Continuous veno-venous hemodialysis (CVVHD) was initiated due to her chronic renal failure with orders to remove 250 mL of fluid per day above baseline. On day 3 of hospitalization she developed decompensated hypoxic respiratory failure and pulseless electrical activity arrest several days later and received 50 minutes of CPR and 20 minutes of chest compressions and shocks. CVVHD was stopped and the patient received 3L of normal saline for volume support. At the time of intubation the patient had copious amounts of pink frothy secretions. Epoprostenol (FLOLAN GlaxoSmithKline, Brantford, UK) was started and the patient required 100% FiO2 and 20 mmHg of positive end expiratory pressure to treat pulmonary hypertension. She was subsequently placed on Artic Sun Protocol; a temperature management system by Medivance (Louisville, CO, USA). This system uses water-circulating gel pads placed on the patient’s skin covering approximately 40% of the patient’s body surface area. This treatment is considered moderate hypothermia and reduces the core body temperature to between 32 °C to 35 °C. After cardiac arrest oxygen stores are depleted and the brain turns to anaerobic metabolism within minutes. This causes cellular trauma which leads to electrolyte imbalance, cytotoxic edema and cell death. Even after oxygen is restored to the brain, inflammation processes continue to injure the brain. This is known as reperfusion injury and may last for up to 48 hours. Induction of hypothermia decreases the cerebral metabolic rate 6 to 7% for every 1 °C drop in body temperature, thereby reducing the inflammatory response and preventing neurological injury.5,6 Additionally, continuous chemical paralytic and sedation infusions were started for five days. Full neurologic recovery was accomplished after 14 days in the ICU. Initial confusion and restlessness was attributed to possible encephalopathy but was more likely related to narcotic withdrawal and ICU psychosis.
DISCUSSION
The genus Serratia consists of at least 15 species that are facultative anaerobic gram-negative rods of the Enterobacteriaceae group. Serratia marcescens is an established human pathogen associated with urinary tract infection, pneumonia, and blood stream infections. Serratia species may be most known for their proportion of eye infections, second only to Pseudomonas aeruginosa. Other common species include S. liquefaciens, S. plymuthica, and S. rubidaea. Human infections caused by Serratia species are thought to arise from exogenous environmental sources rather than from commensal flora. The incidence of Serratia infections is estimated to be 10.8 per 100,000 people annually, with a hospital onset rate of 0.4 per 1000 inpatient discharges according to a single Canadian study.6
To reduce the likelihood of contamination in blood collection, the WHO recommendation for blood drawing is that the skin around the collection site be carefully examined and cleaned before the needle is inserted.7 Skin disinfection reduces the skin bacterial load, but a sterile venipuncture cannot be guaranteed due to inaccessibility of organisms present in sebaceous glands and hair follicles.8
In this case, our blood collection center was notified of the recipient complication and they investigated the donor and the collection process. In their investigation they found that the donor was in good health and the phlebotomy site was acceptable. Although, the exact source of contamination could not be determined, the phlebotomy staff involved was observed to ensure appropriate arm scrub and preparation techniques were followed. It is most probable that the bacterial contamination originated from an environmental source and was introduced into the blood unit during collection or processing. However, the fact that some of the farthest segments tested negative for the bacteria indicates that the contamination most likely occurred from environmental factors during unit processing.
While the exact cause may not be determined, there is a notable learning opportunity in how this case progressed. Roughly an hour after the transfusion was started, it was stopped. It is occasionally stated that allergic transfusion reactions to plasma are an exception to the rule and can be restarted. Transfusions should never be restarted after discontinuation, regardless of the type of reaction. Also, the Transfusion Medicine Service was never consulted during this reaction. It should not be assumed that a reaction is allergic in nature unless discussed with a blood bank supervisor or transfusion medicine faculty member. Restarting this PRBC after being at room temperature for several hours, and subsequently increasing the infusion rate, allowed an additional dose of bacterial contamination to enter the patient’s blood stream and further complicated her clinical picture. A suspected transfusion reaction should always be immediately stopped and reported to the Transfusion Medicine Department. They are most qualified to advise the care team on the best plan of action moving forward. Transfusion reaction signs and symptoms do not always follow the “expected guidelines.” The patient’s vital signs and symptoms in this case could have easily been interpreted as a possible hemolytic, TRALI, or TACO reaction, all of which were mentioned in the patient’s medical record at some point. Symptoms of agitation, feeling uncomfortable, anxious, or emotional (the sense of “impending doom”) should always be taken very seriously and a transfusion should be stopped immediately. These symptoms reported by the patient, are often more critical than vital signs and often implicate a serious reaction.
CONCLUSION
In conclusion, any indication of a possible transfusion reaction warrants investigation by the transfusion medicine service. When there is a suspicion of any reaction, even a mild case, the transfusion must not be restarted but should be sent for additional evaluation and testing in order to prevent a catastrophic event such as this. When blood product contamination is identified, all other products from the donor are quarantined in order to avoid additional complications to other recipients. This case highlights the important role that transfusion medicine practitioners play in maintaining a safe blood supply and ensuring the best outcomes for transfused patients.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONSENT
Verbal consent was taken from the patient.







