OVERVIEW
Chilblain lupus was first described in 1880 by Hutchinson as “pernicious lesions that appear in patients with cutaneous or Systemic Lupus Erythematosus (SLE)”.1 According to the Millard et al series, up to 11.3% of patients with SLE can develop a chilblain lupus.2 At this point, it is necessary to clarify that the term lupus pernio is commonly used to describe a characteristic cutaneous form of sarcoidosis while chilblain lupus is used to describe lupus erythematous lesions.
The prevalence is 3-20% of patients with lupus and, moreover, occurs more frequently in women. It is characterized by purpuric erythematous-violet plaques located in the distal regions, such as the ears, nose, fingers and toes. It has a symmetrical distribution, and they are usually edematous, itchy and sometimes painful lesions. They generally occur as a result of exposure to changes in temperature (cold).
This disorder can appear as an idiopathic dermatosis, usually in young women, but occasionally it is associated with diseases such as anorexia, intestinal lymphoma, chronic myelomonocytic leukemia, monoclonal gammopathies, cryoproteinaemia, viral infections and some connective tissue diseases.3
Chilblain lupus is defined by the Mayo Clinic criteria that include 2 major criteria (dermatological lesions in distal regions induced by cold and histopathological changes of SLE) and 4 minor criteria (coexistence of SLE, other lesions of discoid lupus, improvement with the conventional treatment for SLE and negative results of cryoglobulins and agglutinins). Two major criteria and at least one minor criteria are required to make the diagnosis. The diagnosis must rely heavily on histology, although it is not pathognomonic.4
A differential diagnosis should be made with cutaneous sarcoidosis, erythromegaly, Raynaud’s phenomenon, blue finger syndrome, polycythemia vera and septic embolism, among others. In addition, it is necessary to perform an antinuclear antibodies (ANA) and anti-Ro/SSA antibody detection, for the definitive diagnosis of the patient.
In this clinical case, we observe the evolution from when the patient is diagnosed with chilblain lupus until the final diagnosis of SLE and it’s different systemic manifestations.
CASE REPORT
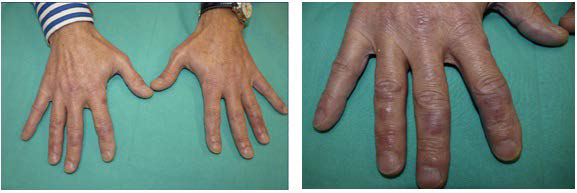
A 61-year-old male patient, smoker and with no other history of interest, came to the Dermatology Department for presenting a three-year history (started in December 2012) of painful and pruritic skin lesions on the back of the fingers with morning stiffness, that appeared in winter and persisted beyond the cold seasons, not sensitive to calcium antagonists and prophylaxis against cold (gloves, avoid exposure, etc) was ineffective. On physical examination, it showed multiple erythematous-violaceous papules and plaques, with some small ulcerations, on the back of the fingers (Figure 1).
Figure 1. Painful, Pruritic and Firm Erythematous-Violaceous Plaques, Located in the Joints and on the Back of the Middle Phalanges
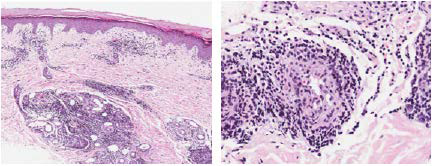
A skin biopsy showed a perivascular infiltrate in the papillary and reticular dermis, as well as a lymphocytic vasculitis, compatible with perniosis (Figure 2).
Figure 2. Skin Biopsy: Superficial and Deep Perivascular Dermatitis with Lymphocytic Vasculitis with Foci of Fibrinoid Necrosis, without Alteration of the Basal Layer of the Epidermis or Presence of Interstitial Mucin, Compatible with Perniosis. (H-E, 10x and 20x)
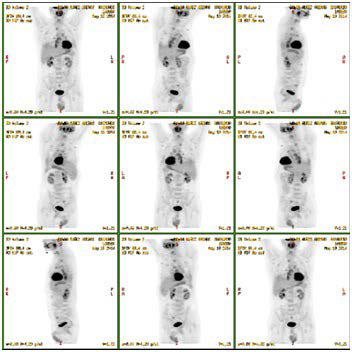
In addition, in April 2013, he was assessed by the Rheumatology Department for reporting arthralgias in both carpus and metacarpophalangeal of both hands, without arthritis. No other physical signs or symptoms of systemic disease were found. Blood tests showed normal renal and hepatic functions, with an increase in acute-phase reactants (ESR 53 mm/h and CRP 2.63 mg/dl), positive antinuclear antibodies (ANA) at titer 1/320, positive anti- Double-stranded deoxyribonucleic acid (DNA) at titer 1/160 and positive anti-Ro and polyclonal hypergammaglobulinemia in the proteinogram, with the rheumatoid factor and cryoglobulins negative. There was no consumption of complements or positive antiphospholipid antibodies. It was decided to expand the work-up after observing a bibasal interstitial pattern on a chest radiograph (Figure 3), so a chest computerized tomography (CT) was performed showing a pulmonary parenchyma with signs of idiopathic interstitial pneumonia, suggestive of usual interstitial pneumonia (UIP) and bilateral axillary and mediastinal pathological adenopathies (Figure 4). Given these findings, positron emission tomography-computed tomography (PET-CT) was performed with the result of slightly hypermetabolic supra- and infra-diaphragmatic adenopathies (Figure 5), therefore a biopsy of an axillary lymphatic node showed an only small granuloma formation and the final results were not suggestive of malignancy.
Figure 3. Chest X-Ray (P-A): Bibasal Interstitial Pattern
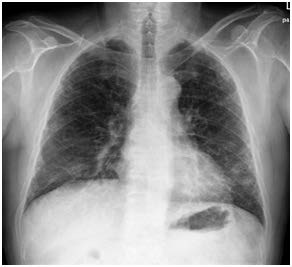
Figure 4. Thoracic CT Scan: Pulmonary Parenchyma with Signs of Idiopathic Interstitial Pneumonia, Suggestive of Usual Interstitial Pneumonia, as there was Linear Thickening of the Interlobular Septa, a Peripheral Honeycomb Pattern with an Apex-basal Gradient and a Slight Infiltrate in the Frosted Glass Distributed Diffusely in Both Bases, Together with Bilateral Sxillary and Mediastinal Pathological Adenopathies
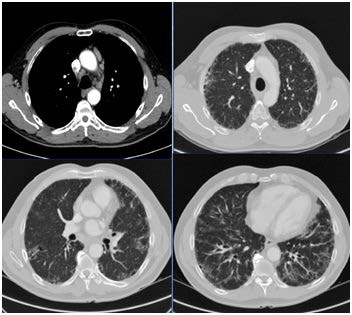
Figure 5. PET-CT: Slightly Hypermetabolic Lymph Nodes Supra and Infra diaphragmatic, Not Suggestive of Malignancy
Based on analysis of all the tests carried out, the Rheumatology and Dermatology departments made the diagnosis of SLE according to systemic lupus international collaborating clinics (SLICC) 2012 criteria with pulmonary involvement (interstitial lung disease), with ANAs and anti-DNA positive antibodies and chilblain lupus was reached, so the patient began treatment with hydroxychloroquine 200 mg twice daily, prednisone 10 mg daily and azathioprine 100 mg daily with partial improvement of skin manifestations and lung disease.
DISCUSSION
Chilblain pernio or perniosis is a rare cutaneous manifestation of SLE that, despite being uncommon, should be followed up when we diagnose erythema pernio. It may go on to develop systemic manifestations of SLE and transform into a systemic disease (chilblain lupus).
From the clinical point of view, the presence of skin lesions compatible with chilblain that persist for a long time and persist beyond the cold seasons obliges us to carry out screening for an associated autoimmune disease. Therefore, complementary tests are needed. It includes the search of laboratory data of SLE,5 as it is in our case, making a definitive diagnosis for presenting tenderness in 2 or more joints with morning stiffness (arthritis), positive ANAs, positive anti-DNA and skin involvement (chilblain lupus), according to SLICC 2012 criteria. In our case, the diagnosis of sarcoidosis was rejected, by the Rheumatology and Dermatology Departments, due to the lack of meeting classification criteria according to the international consensus A Case Control Etiologic Study of Sarcoidosis (ACCESS): granulomas were not observed in the skin biopsy, despite the typical pulmonary involvement of sarcoidosis, and that there was no consistent evidence of the involvement of at least 2 systems or organs for diagnosis.
The skin biopsy, generally, does not offer any help to distinguish chilblain lupus from the idiopathic form. However, at least half of the biopsies of patients with chilblain lupus show some typical histological characteristics of chronic cutaneous lupus erythematosus: interface dermatitis with vacuolation of the basal layer and necrotic keratinocytes with a perivascular dermal infiltrate. On the contrary, the periecrine distribution of the infiltrate is more typical of the idiopathic form.6,7
With the final diagnosis of SLE according to SLICC 2012 (skin involvement, arthritis, positive ANAs, positive antiDNA), it was decided to establish treatment with immunosuppressants for the control of systemic disease.
CONCLUSION
Chilblain is generally a relatively benign disease, but it can sometimes predict a more serious underlying disease, such as SLE, even to present severe pulmonary manifestations, as has been our case, so that treatment with antimalarials and other immune suppressants is recommended.4 In patients with the chronic-onset disease, a careful evaluation is warranted for screening for underlying disease and subsequent clinical follow-up.
CONSENT
The authors have received written informed consent from the patient.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.










