INTRODUCTION
Crohn’s Disease is an autoimmune chronic inflammatory bowel disease associated with both environmental and genetic factors with onset usually in early adulthood.1,2,3 The standard approach to clinical evaluation of the disease involves combining endoscopy findings with radiological and biochemical investigations. For most patients, the disease follows a relapsing and remitting course and thus there is the need for regular follow-up and evaluation of the degree of response to conventional medical therapies, and if this is not the case, for consideration of novel biologic therapies or surgical intervention.4 The disease often manifests within the small bowel although can be distributed throughout any location along the gastrointestinal tract.3 Therefore, the absence of visible disease during a colonoscopy cannot exclude small bowel involvement. A previous endoscopic study of the small bowel revealed that disease is indeed present there amongst 65% of Crohn’s patients.5 Therefore, information from faecal biomarkers and radiological investigations taken into consideration in conjunction with colonoscopy findings when assessing the comprehensive pattern of disease amongst individual patients.6
Previously other markers of disease activity have been investigated, such as C-reactive protein (CRP). CRP is a traditional and well-recognised sensitive marker of the degree of inflammatory activity within the bowel. However, as has been described to also be the case for erythrocyte sedimentation rate (ESR) and platelet count, CRP is not specific for Crohn’s.1,6
Faecal Calprotectin as a Biomarker of Inflammation
Calprotectin is a 36-kilodalton protein that is present in plasma but also understood to be concentrated in stool samples. In physiology, the protein is not normally found in gut mucosa.7 However, in pathophysiological states such as within the inflammatory lesions of Crohn’s disease, neutrophils migrate through the bowel wall and reach the mucosa. This protein is then released from the cytoplasm of granulocytes. As the protein remains intact in stool samples for up to 7 days, calprotectin is an investigation that has become widely used in a range of clinic or general practice settings as a surrogate marker of inflammation in inflammatory bowel diseases.8,9 It is understood, however, that increased levels of faecal calprotectin may represent the presence of inflammatory processes within the intestine from a broad range of aetiologies.1 Defining the presence versus absence of inflammation has been of particular clinical value in one group of patients; differentiating between patients with inflammatory bowel disease (IBD) and those with non-IBD diagnoses, such as irritable bowel syndrome (IBS). A 2007 quantitative meta-analysis of 35 studies evaluated the role of faecal calprotectin in this diagnostic dilemma and found the test to be effective in discerning patients with Crohn’s disease from those with IBS.10 Further studies have supported calprotectin as a more reliable marker of inflammation than ESR and CRP. Tibble et al6 included a comparison of CP, ESR and CRP for differentiating between Crohn’s and IBS. CP was found to have a 100% sensitivity and a 97% specificity when a cut-off point of 30 mg/l was used, and that the diagnostic accuracy of calprotectin was superior to ESR and CRP.6 Early studies such as these utilised 111 indium marked white cells as a gold standard, related to its validated ability to represent intestinal inflammation.11
A 2010 meta-analysis sought out to answer a similar clinical question to that posed by ourselves: whether the faecal calprotectin levels could be used to improve patient selection for colonoscopy following presentation with clinical features suggestive of Crohn’s. The aim was to determine whether colonoscopy could be safely omitted in the diagnostic evaluation of low risk patients assessed as such based on the presence of this biomarker at lower-levels. Six prospective studies in adults were included in the analysis. The study found a sensitivity of 93% (95% CI, 0.85 to 0.97) for calprotectin in detecting inflammatory disease later confirmed by colonoscopy. The specificity was found to be 93% (95% CI, 0.79 to 0.99). The authors concluded that by using calprotectin levels in patient selection, there could potentially be a 67% reduction in colonoscopy procedures.8
The Crohn’s disease activity index (CDAI) is measure of disease activity through clinical assessment. This index, together with serological and faecal inflammatory markers, is taken into consideration by the clinician when holistically assessing disease activity and response to treatment during follow-up.2 Costa et al who found significantly higher-levels of calprotectin amongst patients with higher clinical disease activity scores.12 An increasingly adopted therapeutic target is the attainment of ‘mucosal healing’. This can be assessed through both endoscopy and radiological investigations.13
Findings from a recent study using double-balloon enteroscopy (DBE) to compare the accuracy of inflammatory markers in the detection of disease activity and mucosal healing have further supported the role of calprotectin as a sensitive marker of disease activity.7 Calprotectin demonstrated a stronger correlation (r=0.77; p=0.001) with disease activity when compared to CRP (r=0.65; p<0.001) or to platelets (r=0.49; p<0.001) and serum albumin (r=-0.47; p=0.001).7 Notably, the authors concluded that calprotectin was a particularly useful marker of disease regression in those patients with Crohn’s who had involvement of the ileum. It could be argued that the outcome of this study backs up a potential role for calprotectin to be used as a cut-off for reducing the burden of unnecessary MRI small bowel scans. This study also demonstrated that despite its widespread clinical use, CRP did not perform as well as calprotectin and was only increased in Crohn’s patients with more moderate to severe disease activity.7
Limitations of Colonoscopy in Crohn’s
Colonoscopy has the advantage of providing a direct visualisation of the colonic mucosa and facilitating biopsy of lesions. It is therefore very useful in investigating patients presenting with symptoms suggestive of an inflammatory process within the bowel. However, colonoscopy can only visualise the colon up to the terminal ileum and therefore is limited in evaluating the pattern of disease involvement in Crohn’s.14 In addition, there are rare but significant potential complications associated with the procedure such as bowel perforation. Colonoscopy is also associated with considerable procedural discomfort for patients. Attempts at use of ultrasound for this scenario are limited by the presence of intervening bowel gas.15
Computed tomography enterography (CTE) is another cross-sectional imaging modality to investigate Crohn’s disease. A 2012 study using this modality amongst 153 patients undergoing colonoscopy with intubation of the terminal ileum found that up to 54% of patients with small bowel or with upper gastrointestinal involvement were found to have a normal terminal ileum on endoscopy. The authors also suggested that in some cases disease evident on cross-sectional imaging may evade visualisation under colonoscopy because of a pattern of intramural or mesenteric involvement. In many other cases, the absence of disease was attributable to the more widely recognised explanation of the disease skipping the terminal ileum. This study bolsters the application of cross-sectional imaging in investigating patients with clinical features of Crohn’s disease but with a negative colonoscopy.16
Capsule endoscopy has a significant role in the evaluation of small bowel lesions beyond the reach of the endoscope; however, is also limited in that it cannot be used with the presence of structuring disease in the small bowel, which is common in Crohn’s.14
Double-balloon enteroscopy (DBE) has been demonstrated to have a role in identifying inflammatory lesions in the ileum and other parts of the small bowel beyond the reach of conventional colonoscopy; however, the use of this method is confined to small-scale use in specialist centres and is not routinely used in clinical practice due to the risks involved and duration of procedure.3,7
Role of MRI in Assessment of Small Bowel Disease
Over the last few years there has been an increasingly widespread adoption of cross-sectional imaging modalities such as MRI in the evaluation of Crohn’s disease. CTE is utilised to a lesser extent due to the considerable level of exposure to ionising radiation involved. MRI also has the advantage of superior soft tissue contrast resolution when compared to computed tomography (CT) and multiplanar capability3; however, CT has better spatial resolution.14,15 MRI necessitates the use of adequate oral contrast for the purposes of distending the bowel in order to better visualise areas of enhancement.15 Furthermore, whilst endoscopy has the ability to assess mucosal surface disease, MR has the ability to evaluate the extent of involvement of active inflammation through the thickness of intestinal wall, and investigate both intramural and extramural disease.3,4 In addition, MRI has the ability to evaluate the structural complications of Crohn’s such as fistulas and abscesses.2 MRI is also able to detect lymphadenopathy.3 A key factor behind the burgeoning demand for MRI is its ability to both visualise bowel segments proximal to strictures which may be beyond the reach of colonoscopy.3 Thus better spatial resolution and the improvements in techniques to reduce artefact secondary to bowel peristalsis have led to a more widespread adoption of MRI in assessment of small bowel pathology.13
Radiological findings suggestive of active inflammation include mural signal intensity, degree of enhancement with gadolinium contrast and mural thickness. Mural thickening has been found to correlate with CDAI, a disease score based on clinical and laboratory data.14
MRI has been validated in the assessment of Crohn’s through a number of studies such as Rimola et al, which correlated MRI lesion marker of severity with their corresponding visualised lesions on endoscopy.15 In this study, the two investigations were performed during the same day. Endoscopic assessment of severity of inflammatory lesions was undertaken with the Crohn’s disease endoscopic index of severity (CDEIS). This scoring system takes into account features such as the presence of deep or superficial ulcers and the presence of luminal bowel stenosis along the length of the colon through to the terminal ileum. The study looked at patients with an established diagnosis of Crohn’s and found that there was a close relationship between the severity of endoscopic lesions and MRI findings associated with severity. The most suggestive MRI finding was oedema of the wall, which was found in 77.5% of ulcerated segments on endoscopy, and this was not present in any regions of the colon identified as normal on endoscopy.15 The sensitivity of MRI was increased when multiple radiological findings such as relative contrast enhancement were considered together with wall thickening. Such MRI findings had a high diagnostic accuracy. The study suggested specifically that in patients with known Crohn’s, MR could be used as an alternative to colonoscopy to provide a complete assessment of the colon for disease.15 In addition, it has been observed that there is a decrease in MRI findings such as contrast enhancement of the bowel wall in patients who progress from active disease to disease in remission, and that MRI is useful for the follow-up of patients with Crohn’s.2
Del Vescovo et al identified that with the use of dynamic contrast enhanced MR of the terminal ileum, MRI findings correlated well with histological confirmation (r=0.8; p<0.001, Spearman test). It was found that this more modern imaging technique was associated with an increased accuracy in differentiating inactive from active disease.4
Whilst MRI is widely used in excluding inflammatory bowel disease in patients who present with enteric symptoms, the method is limited in its ability to detect early stages of Crohn’s, such as mucosal nodularity and superficial aphthous ulceration.3
METHODS
A total of 422 MRI small bowel study scans carried out at a large district general hospital, Stevenage from between the dates of 02/04/2015 and 21/07/2017 were analysed. One hundred nineteen of these were excluded as there was no information regarding scan reports and/or faecal calprotectin levels for these patients. Of the remaining 301 scans, 21 (6.9%) scans were excluded as they were for indications other than the investigation of Crohn’s disease. One hundred seventy-four (57.8%) scans were carried out to investigate the extent of disease activity or the degree of remission in patients with known Crohn’s disease. Only 78 scans were included as they had a recent calprotectin taken prior to scan (<4 months).
One hundred six (35.2%) scans had been undertaken to investigate the presence of small bowel involvement within patients who had presented with symptoms suggestive of Crohn’s disease. From this cohort 85 patients who underwent MRI small bowel study and had a normal colonoscopy prior to scan were included.
In the statistical analysis applied to the cohort of patients who presented with symptoms suggestive of Crohn’s but did not have a prior diagnosis, we define a ‘true positive’ test result as an MRI demonstrating the presence of disease in a patient who had a calprotectin level of greater than 600. Similarly, a ‘true negative’ test result refers to an MRI scan which did not demonstrate any significant evidence of disease in a patient who had a calprotectin level below 600. The ‘sensitivity’ of calprotectin therefore reflects the performance of this biomarker in correctly identifying all those patients who are found to have evidence of small bowel disease on MRI. Similarly, the ‘specificity’ of calprotectin relates to the performance of this test in identifying patients in whom small bowel involvement is found to be absent on subsequent MRI.17
Furthermore, the positive predictive value (PPV) of the test reflects the likelihood that the patient has an MRI scan demonstrative of significant disease, given the calprotectin level is above 600. The negative predictive value (NPV) of the test refers to the likelihood that the patient has an MRI scan reporting the absence of any significant disease if the calprotectin level is below 600.
The statistical analysis was similar in the cohort of patients with an established diagnosis of Crohn’s, however, in this group of patients, sensitivity and specificity relate to the performance of this test in identifying the presence or absence of active disease respectively.
RESULTS
Calprotectin Level as a Cut-off for MRI Investigation of Patients with Symptoms of Crohn’s Disease
Eighty-five patients who presented with symptoms suggestive of Crohn’s disease and then had a normal colonoscopy were studied (Figure 1). When looking at the 55 of these patients who had a calprotectin below the cut-off value of >600, we found that none (0%) of these patients were found to have significant evidence of Crohn’s disease on the subsequent MRI scan. Of the 30 patients who did have a positive calprotectin, 8 (26.6%) of these were found to have significant disease on MRI. The sensitivity of calprotectin was therefore identified to be 100% (95% CI, 63.06% to 100%) and the specificity of the test was found to be 71.43 % (95% CI, 60% to 81.15%). The NPV was calculated to be 1.00. The PPV was found to be 0.27.
Figure 1: Histogram to Show Distribution of Calprotectin Levels in MRI +ve and MRI -ve Groups
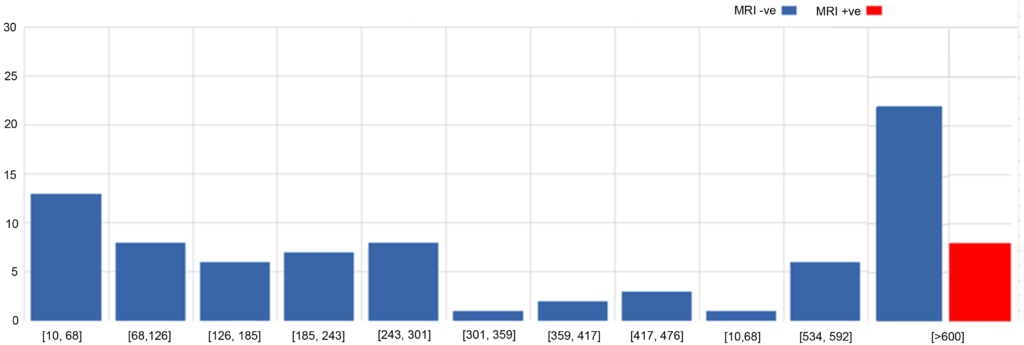
Calprotectin as a Cut-off for Investigating State of Disease Activity/Remission in Patients with known Crohn’s Disease
Of the 40 patients with known Crohn’s disease who have a recent (<4 months) calprotectin that was positive and then underwent MRI to investigate disease activity, 25 (62.5%) of these had a scan demonstrative of active disease. Of the 38 patients with known Crohn’s disease who have a negative recent (<4 months) calprotectin who underwent MRI to investigate disease activity/extent of remission, 16 (42.1%) of these had a scan demonstrative of active disease. The sensitivity of a positive calprotectin for the presence of active disease amongst patients with known Crohn’s was found to be 60.9% (95% CI, 44.5% to 75.8%) and the specificity 59.5% (95% CI, 42.1% to 75.3%) (PPV 0.63, NPV 0.58).
DISCUSSION
The Royal College of Radiologists (RCR) reports that there are ever increasing workload demands on radiology services as a result of a low radiologist to population ratio, and an increasing burden of reporting lists on that limited number. Overall, the number of radiological investigations has increased by 42% in the last 10 years. Of particular note is the fact that the largest area of growth in radiological investigations carried out is likely to be in MRI, which is projected to amount to a number of 7.8 million in 2022.18 There is therefore need for studies evaluating novel approaches to reduce the burden of inappropriate examinations.
To the best of our knowledge, we are the first authors to investigate the performance of a calprotectin cut-off level within the specific role of selecting patients (who have had a normal colonoscopy) for MRI with the intention of reducing the burden of unnecessary scans carried out in the radiology department of a district general hospital. Our data has demonstrated that within the 55 patients who had a normal prior colonoscopy with a calprotectin of less than 600, none of these were found to have significant evidence of small bowel involvement on the subsequent MRI scan. This in theory suggests a 100% sensitivity of the biomarker at this level. We believe that if our findings are corroborated with further prospective studies on the use of calprotectin in patient selection for MRI, use of this marker may be a viable approach to reduce such unnecessary MRI examinations. Evidence from prior studies, some of which described above, suggests calprotectin is a sensitive but non-specific investigation for the presence of inflammatory bowel disease. As corroborated by the previous data, our data suggests by comparison a lower specificity for the investigation identifying the presence of disease, at 71.43 % (95% CI, 60% to 81.15%).
In our study we have additionally attempted to investigate the use of calprotectin levels in patient selection for MRI small bowel scans that were requested for investigating active disease in patients with known Crohn’s. This is a likely source of increased demand on MRI over the coming years given the expansive use of biological therapies such as ustekinumab and adalimumab. There is the need to characterise the extent of small bowel involvement whilst selecting patients for these therapies and monitoring the degree of radiographic response once therapy has been initiated.19 It may be that further studies validating the use of calprotectin as a cut-off may have considerable economic benefits in reducing the need for MRI and perhaps itself having a direct role in patient selection for biological therapies.
This study found similarly that calprotectin was less sensitive 60.9% (95% CI, 44.5% to 75.8%) in this context of identifying patients with known Crohn’s who would demonstrate the presence of active disease on MRI. In addition, the test was less specific for this outcome, at 59.5% (95% CI, 42.1% to 75.3%). It should be noted that this aspect of the study was limited by the use of calprotectin data within a broad time period of 4 months prior to the scan. During the intervening time period in between the positive calprotectin and the scan, changes to a medical therapy regimen would have resulted in reduced active disease on the subsequent MRI scan. Further prospective studies investigating such a role of calprotectin may include calprotectin levels obtained immediately prior to scan.
CONCLUSION
Our study is the first to suggest that in patients with clinical features of Crohn’s disease who have a normal colonoscopy, calprotectin performs impressively as a sensitive marker of the presence of small bowel inflammation on subsequent MRI. There could therefore be significant clinical utility for this biomarker in deciding whether such patients need to undergo further small bowel imaging following a colonoscopy. Further higher-powered prospective studies would be merited to investigate this potential use in the clinical setting and therefore if suitable its application in reducing the growing burden of MRI requests to the radiology department and sparing patients of unnecessary investigations.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.






