INTRODUCTION
I t is well-recognized that overweight and obesity are associated with impaired glucose control.1 For healthy individuals, a normal fasting blood glucose level is between 70 and 100 mg/dL, and a normal post-prandial blood glucose level is between 100 and 140 mg/dL. In people with high body weight, insulin resistance develops due to excess adipose tissue resulting in the dysregulation of blood glucose levels.2,3 Glucose is the primary fuel in the body for energy production in the cells, especially in the brain and red blood cells.4,5 Glucose molecules circulate in the bloodstream supplying energy to the cells until extra glucose molecules are taken up by the tissues for storage.5 Glycemic control is defined as the overall adequacy of which the body regulates blood glucose within a normal range. Glucose homeostasis is regulated primarily by glucoregulatory hormones insulin and glucagon.6 In muscle, insulin promotes glucose uptake and protein synthesis, in adipose tissue insulin promotes glucose and fatty acid uptake and inhibition of lipolysis, and in the liver insulin promotes glucose utilization and suppresses glucose production.7 Glucagon facilitates the release of glucose from stored glycogen in the body to increase glucose concentration in the blood.5,8 These hormones act in a negative feedback loop regulating one another. Disruptions in the signalling of these hormones, physical damage to the cells involved, and decreased effect of these mechanisms can all lead to glucose dysregulation. In overweight and obesity, body tissues become less sensitive to the effects of insulin resulting in decreased uptake of glucose from the bloodstream.1 Furthermore, the presence of insulin does not properly inhibit glucagon action on the liver resulting in continued hepatic glucose output despite adequate glucose already present in the blood.9 Impaired glycemic control is associated with disturbances in the metabolism of all three macronutrients, damage to vascular tissue including blood vessels, and disruption of organ function, all of which can then lead to chronic health conditions, such as cardiovascular disease, stroke, and type 2 diabetes (T2D).5,10,11 Glucose dysregulation results in aberrations of lipid metabolism, which increases risk of atherosclerosis and cardiovascular disease.11,12 Studies indicate there is a direct relationship among blood glucose, lipid metabolism, and pancreatic beta cell dysfunction.10 Soluble fiber has been shown to improve glycemic control by slowing the absorption of glucose in the gastrointestinal (GI) tract thereby decreasing the glycemic response.13 The effect of psyllium as a soluble fiber on glycemic control has been widely researched and has shown significant positive results.14,15,16 Inositol, an organic stereoisomer of glucose, is present in all mammalian tissues and is found naturally existing in fruits, grains, beans and nuts.17 Inositol plays a role in various biological processes such as signal transduction, cell growth, phospholipid synthesis, osteogenesis, reproduction, etc.18 It has been identified as a factor in the insulin signaling pathway that regulates blood glucose levels and has been implicated as beneficial to glycemic control when taken as a dietary supplement in T2D, gestational diabetes, polycystic ovary syndrome, and metabolic syndrome.19,20 Currently there is little research on the effects of inositol supplementation on glycemic control in those who are euglycemic or have pre-clinical hyperglycemia, or the effects of combining inositol with other dietary supplements such as soluble fiber.
The purpose of this study was to investigate first, the efficacy of supplementation of inositol by itself on glycemic control in overweight and obese subjects with pre-clinical impaired glucose control and second, the effects of supplementation of inositol in combination with soluble fiber. Due to coronavirus disease-2019 (COVID-19), only one overweight subject completed both phases of supplementation. The results from the subject demonstrated that supplementation of 4 grams of myo-inositol daily resulted in improved glucose parameters and lipid parameters including fasting blood glucose, post-prandial blood glucose, total blood cholesterol level, blood high-density lipoprotein (HDL) cholesterol level, and blood triglyceride level in the overweight subject. The combination of inositol and soluble fiber supplementation further improved the total blood cholesterol level although the synergistic effects were not seen for other parameters. These results indicate there is potential benefit of inositol supplementation for sub-clinical hyperglycemic overweight subjects on glycemic control and on control of blood lipid levels.
MATERIALS AND METHODS
Participants and Screening
The study was performed from January through March 2020. Overweight and obese men and women of all ethnic and racial groups aged 18-years or older with body mass index (BMI) equal to or greater than 25 kg/m2 were recruited from the St. Louis, Missouri, USA metropolitan area by way of verbal interactions, hardcopy flyer distribution, and social media recruitment on Facebook. Volunteers were pre-screened by email using a potential participant questionnaire to determine provisional eligibility. This questionnaire assessed age, height, weight, medical history and current medication(s)/supplement(s). If volunteers met the stated criteria, they were scheduled for a formal on-site screening in the Physiological Laboratory at Doisy College of Health Sciences, Saint Louis University. At this time, the participants signed an informed consent document and Health Insurance Portability and Accountability Act (HIPAA) form approved by the Institutional Review Board (IRB) of Saint Louis University (Protocol ID # 30729). After obtaining of informed consent, the official screening was conducted by measuring body height, weight and fasting blood glucose levels. Fasting blood glucose levels were measured using blood samples obtained via finger stick method.21 Inclusion criteria included a calculated BMI equal or above 25 kg/m2. A fasting blood sugar greater than or equal to 126 mg/dL, the presence of any significant medical condition including a medical diagnosis of diabetes mellitus or taking any medications and/or dietary supplements that may interfere with glucose or lipid regulation were cause for exclusion.
Study Design
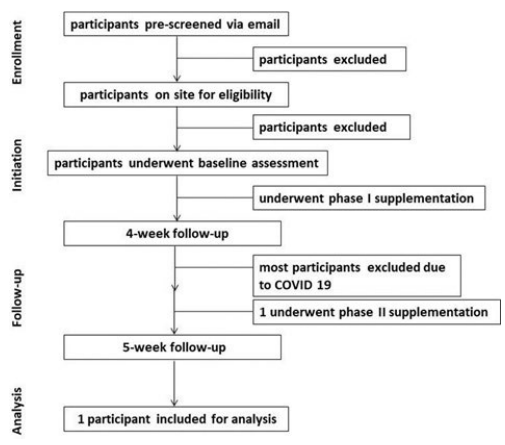
The study followed a single cohort, uncontrolled, test-retest design. The study lasted 5-weeks and included two phases of supplementation. The first phase lasted 4-weeks and the participants supplemented 2 grams of myo-inositol twice daily. The second phase lasted 1-week, and the participants supplemented 2 grams of myo-inositol plus 2 grams of soluble fiber each twice daily. The participants underwent 3 data collections including baseline assessment, 4-week follow-up assessment, and 5-week follow-up assessment (Figure 1).
Figure 1. Flow Diagram of the Study. The Diagram Indicates the Flow of the Study
Body Mass Index Calculation
BMI was calculated by using the equation BMI=kg/m2. Body weight of human subjects was measured in pounds using a standard balance scale and body height was measured in centimetres with the height rod attached to the scale. Height in centimetres were converted to meters and weight in pounds were converted to kilograms.
Glucose Tolerance Test
A modified glucose tolerance test was used to measure glycemic control of the participants.22 The test was completed after overnight fasting. Participants drank a 10-ounce glucose drink containing 50 grams of dextrose (Thermo Fisher Scientific, Cat #: 401272P). The post-prandial measurements were taken exactly one hour after the drink was finished.
Analysis of Blood Samples
All blood samples were collected by finger stick method using disposable spring activated lancets. Approximately 15 μL of blood were used for each measurement. Analysis of all blood samples was conducted using a CardioChek (PTS Diagnostics, Cat #: 1708) to measure blood glucose concentration (mg/dL), total blood cholesterol concentration (mg/dL), blood HDL cholesterol concentration (mg/dL), and blood triglyceride concentration (mg/dL).
Nutrition Supplementation
It is considered very safe to supplement soluble fiber and inositol. Both soluble fiber and myo-inositol can be obtained without a prescription. It is recommended to orally take soluble fiber and myo-inositol up to 20 grams and 18 grams per day, respectively.23 The supplement regime was broken into two phases. During Phase 1, lasting four-weeks, all participants were supplemented with 2 grams of myo-inositol orally twice daily. During phase 2, lasting one week, all participants were supplemented with 2 grams of soluble fiber orally twice daily in addition to the inositol regimen. Supplements were distributed at the beginning of each phase.
Participants were instructed verbally on the supplement regimen and given clearly written supplement instructions to take with them. To avoid reduction of inositol absorption by soluble fiber, inositol needed to be taken 1-hour before meals and soluble fiber needed to be taken after the meals. Supplements were counted and the exact number of intended supplements to take were distributed to each participant. Participants were instructed to return any remaining supplements at the end of each phase in order to account for missed doses. Supplements included Premium Supplements myo-inositol capsules (Norax Supplements, Newnan, GA, USA) and Equate Daily Fiber Capsules (Walmart, Cat #: 5570980260). A supplement log was distributed to all the participants in order to keep track of supplement intake and returned at the end of each phase.
Statistical Analysis
A statistical power analysis using G*Power Software (version 3.1.9.4) to calculate the sample size was performed based on the following inputs: t-tests (Means: Difference between two dependent means (matched pairs), alpha=0.05, desired power=0.70.24 The results indicated that a total sample size of 12 would be required to detect large effects of supplementation (d=0.8). However, since only one participant completed the whole study due to COVID-19, the study is presented as a case study.
RESULTS
Study Participants
The flow diagram demonstrates the flow of the study participants (Figure 1). Due to COVID-19, only an overweight subject was able to complete both phase 1 and 2 supplementation. This participant was a 49-year-old female with a height of 168 cm, a weight of 78.6 kg, and a calculated BMI of 27.9 kg/m2 (Table 1). The participant also indicated no presence of any significant medical condition including a medical diagnosis of diabetes mellitus and did not take medications and/or dietary supplements that may interfere with glucose or lipid regulation. In terms of number of doses throughout the study, the adherence to the supplements was 99% since the participant only missed one dose of 2 grams myo-inositol. Therefore, the results of this study from the only one participant are reported as case study to present the valuable data without inferential statistical analysis.
| Table 1. The Demographics of the Subject |
|
Participant
|
Gender
|
Age (year) |
Height (cm) |
Weight (kg) |
BMI (kg/m2)
|
| #3 |
Female
|
49 |
168 |
78.6 |
27.9
|
| BMI: body mass index |
Glucose Parameters
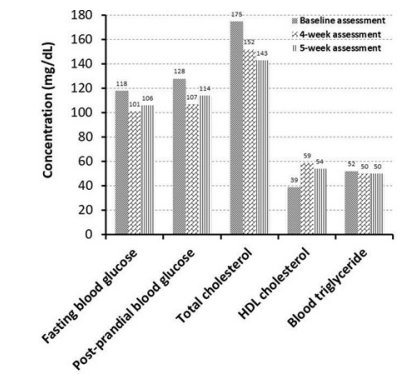
A decrease in both fasting blood glucose level (-14.4%) and postprandial glucose level (-16.4%) were observed from baseline to the four-week point. Fasting blood glucose concentration decreased to 101 mg/dL from118 mg/dL after supplementing 2 grams of myoinositol twice daily after phase 1 supplementation. Post-prandial blood glucose (after the 50 grams dextrose drink) decreased from 128 mg/dL at baseline to 107 mg/dL after phase 1 supplementation (Figure 2, Table 2). However, the further improvements were not observed after phase 2 supplementation. At the five-week follow-up, fasting and post-prandial blood glucose concentrations rose to 106 and 114 mg/dL respectively, (Figure 2, Table 2). These values were increased from the four-week values. However, they were still improved from the baseline numbers.
Figure 2. The Effects of Inositol and Soluble Fiber Supplementation on Blood Glucose Levels and Other Parameters
| Table 2. The Measurements of Blood Glucose Levels and Other Parameters |
| |
Baseline Assessment
|
4-week Assessment |
5-week Assessment
|
| Fasting blood glucose (mg/dL) |
118
|
101 |
106
|
| Post-prandial blood glucose (md/dL) |
128
|
107 |
114
|
| Total cholesterol (mg/dL) |
175
|
152 |
143
|
| HDL cholesterol (mg/dL) |
39
|
59 |
54
|
| Triglyceride (mg/dL) |
52
|
<50 |
<50
|
| BMI: body mass index |
Fasting blood glucose, post-prandial blood glucose, total blood cholesterol, HDL cholesterol, and
blood triglyceride levels were measured at the start of the supplementation (Baseline assessment), after supplementation of only inositol for 4 weeks (4- week assessment), and after supplementation of inositol plus soluble fiber for additional one week (5-week assessment).
Lipid Panel
Improvements in total cholesterol, HDL cholesterol, and triglyceride were seen from baseline to four weeks. Total cholesterol decreased from 175 mg/dL to 152 mg/dL after phase 1 supplementation, and then even further decreased to 143 mg/dL after phase 2 supplementation (Figure 2, Table 2). HDL cholesterol increased from 39 mg/dL at baseline to 59 mg/dL after phase 1 supplementation: however, a slight decrease was seen after phase 2 to 54 mg/dL (Figure 2, Table 2). A decrease in serum triglycerides was observed from baseline (52 mg/dL) to below 50 mg/dL after both phase 1 and phase 2.
DISCUSSION
Our results indicate that intervention with inositol to improve blood glucose regulation in the target population can be effective and therefore may prolong progression to prediabetes or T2D. Furthermore, improved glycemic parameters in this population can positively impact lipid parameters consequently. Glycemic control is one indicator of metabolic health. Overweight and obese are associated with the loss of glycemic control primarily through insulin resistance.1 However, glucose regulation is a complex mechanism involving many different cell types, hormones, secondary compounds such as inositol, and lifestyle factors like diet and exercise. Because inositol is known to play a role in the insulin signaling pathway, and that dietary supplementation of inositol has been shown to improve glycemic control in conditions such as diabetes, we proposed that supplementing 4 grams of inositol daily for 4-weeks would result in improved glucose parameters. As soluble fiber decreases glycemic response by slowing absorption of exogenous glucose, supplementing 4 grams of soluble fiber daily in combination with the 4 grams of inositol would have even greater effects on these parameters due to a synergistic effect. Due to COVID-19, full data were only collected on one subject. The data collected from one subject is interpreted as a case study involving supplementation of inositol and soluble fiber on glucose and lipid parameters. If we interpret the results obtained from the only completed participant at face value, the correlation displayed between inositol supplementation and improved blood glucose parameters aligns with related studies as well as our hypothesis for this study. Inositol has shown benefits in glycemic control of T2D, and because overweight status is associated with pre-clinical hyperglycemia, we expected the same effects would occur in people of this population. As mentioned, previously, the subject studied was in the prediabetic range at baseline based on fasting blood glucose concentration. After phase 1 of supplementation the participant’s fasting blood glucose level was 101 mg/dL which is only 2 mg/ dL above normal, indicating the participant was almost out of the prediabetic range. Furthermore, the subject had an HDL cholesterol level of 39 mg/dL at baseline, which was considered dyslipidemia.5 At the 4-week point, HDL cholesterol increased to within normal range. Clinically, these results are very significant. These changes not only indicate improvement in glycemic control but decreased risk for developing T2D and cardiovascular diseases. If these results were replicated in a larger population of people who are prediabetic, then inositol supplementation may become one strategy for prevention of progression from prediabetes to diabetes. Combined supplementation of inositol and soluble fiber did not have the added benefit for most parameters that we expected. Inositol and soluble fiber supplements have different mechanisms of action on glycemic control. Inositol promotes glucose transporter type 4 (GLUT4) translocation to the cell membrane facilitating glucose uptake and soluble fiber binds to glucose in the GI tract slowing its absorption.17 We hypothesized these mechanisms would have added benefit; however, the results did not reflect this hypothesis for most parameters. This could have been caused by reduced absorption of inositol by soluble fiber if the supplements were not taken far enough apart. That’s why participants were instructed to take inositol one hour before meals and soluble fiber right after the meals. As a case study with one subject, one of the limitations is the great variance in dietary factors and physical activity. These factors may have a direct impact on blood glucose and lipid levels therefore could play a role in the change observed from baseline to follow-up measurements. And the purity and potency of nutritional supplements are not regulated by the Food and Drug Administration (FDA) as food and drugs are. Variations in the myo-inositol and soluble fiber capsules could be an unknown outlier in this study.
CONCLUSION
In conclusion, results from this study indicate inositol supplementation improves glycemic control and blood lipid profile, and supplementation of inositol plus soluble fiber further improved the total cholesterol level. These findings indicate that there is potential benefit of inositol supplementation for the sub-clinical hyperglycemic, overweight population on glycemic control, and that further research on these effects through future studies is warranted.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the participants who volunteered to this study, especially, the participant who completed the whole study. The authors also would like to thank Drs. Uthayashanker Ezekiel and Edward Weiss for their valuable suggestions regarding the study design and data analysis and thank Sabrina Hollar for assistance in editing the manuscript.
FUNDING
This work was supported by start-up funds from Saint Louis University to YL.
ETHICAL STATEMENT
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Institutional Review Board of Saint Louis University (Protocol ID # 30729). Written informed consent was obtained from all subjects.
AUTHOR’S CONTRIBUTION
HS and YL conceived and designed the study, interpreted the results, wrote the manuscript; HS conducted the study. All authors have critically reviewed the manuscript.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.







