INTRODUCTION
Over the years, anthropogenic activity have led to accumulation of several heavy metals in the environment, causing serious health problems in humans. Cadmium (CD) is considered one of the most common and most dangerous environmental pollutants of the natural and occupational environment in industrialized countries all over the world.1 Continuous CD release from natural sources, i.e., the Earth’s crust, and various human activities, like industrial processes, has led to an increase in CD concentrations in the environment. Increased CD exposure has increased throughout the population for the lifetime of the individual. Heavy metals exhibit a density that is at least five times greater than that of water and isn’t biodegradable. The long biological half-life leads to an accumulation in different organ systems, leading to undesirable side effects and are relatively more toxic even at very low concentrations.2 CD is a heavy metal widely utilized in industry, affecting human health via occupational and environmental publicity.3 CD may be absorbed and accrued in flora and animals through water, air, and soil and, consequently, within the human body through the food chain and is a major source of human exposure with the liver and kidney affected.1,3 Studies have shown that exposure to CD causes haematological, hepatotoxicity, neurobehavioral parameters disorders. CD-related disorders are associated with CD toxicity in liver and affect cell proliferation, differentiation, apoptosis, and other cellular activities. Cellular changes include the swelling of hepatocytes, fatty changes, focal necrosis, and hepatocytes degeneration, all considered markers of impaired function.3 The role of certain metals and vitamins in modulating the effects of different toxicants is an area of recent interest. Vitamins are essential to maintain normal metabolic processes and homeostasis within the body. Vitamin C and Vitamin E are low molecular mass antioxidants that scavenge or quench free radicals.4
Natural antioxidants, Vitamins A, C, E and carotenoids, are derived from food and are consumed through diet.5 Recently, much interest has been given to the role of natural antioxidants as prevention against oxidative damage as a factor in the pathophysiology of various health issues.6 Among the antioxidants ascorbic acid (Vitamin C) and Tocopherol (Vitamin E) used as a nutritional supplements and are considered essential elements in almost all biological systems. Vitamin C is a water-soluble chain-breaking antioxidant and can scavenge superoxide and hydrogen peroxide.7,8 Vitamin E (α-Tocopherol) is a lipid soluble vitamin with powerful biological antioxidant.7,9 Zinc is an essential trace element in men, relatively non-toxic,10 ubiquitous in subcellular metabolism and an integral component of catalytic sites of enzyme classification.11 To date, the full impact of the environmental contamination has not been elucidated in the biological, where the effect of heavy metals including CD either individually or in combination with natural antioxidants may require a thorough examination to understand the interaction between CD with antioxidants. The present study was performed to determine the amelioration capabilities of Vitamin C, E and Zinc in wistar rats from CD-induced toxicity.
MATERIALS AND METHODS
Animals
Male rats weighing 225±10 g were selected in the present study and were housed in stainless steel mesh cages, under standard laboratory conditions (Temperature 23±2 °C, 50±10%; Relative humidity, 12:12 Light: Dark cycle). The animals were fed with standard rat chow (obtained from Sai Durga Feeds and Foods, Bangalore, India) and drinking water ad libitum. The rats were acclimatized to the laboratory conditions for ten days. The Institutional Animal Ethics Committee has approved the Experimental protocols and animal use (Resol. No. 60b/2012/(i)/a/CPCSEA/IAEC/SVU/MSR–RS dt. 08.07.2012), Sri Venkateswara University, Tirupati, Andhra Pradesh, India.
Chemicals
Cadmium as Cadmium chloride (CdCl2), Zinc as Zinc chloride (ZnCl2), Vitamin C (Ascorbic acid), and Vitamin E (α-Tocopherol) was obtained from Sigma Chemical Co, Loba Chemicals and SD Fine-Chemicals, Maharashtra, India. All the chemicals used in this study were of the highest purity.
Experimental Design
Rats were divided into 8 groups; each contained 6 rats and fed one of the following diets.
Group 1: Control group
Group 2: CdCl2 dissolved in drinking water @ 10 mg/L
Group 3: CdCl2 (10 mg/L of drinking water)+Vitamin C (100 mg/kg BW)
Group 4: CdCl2 (10 mg/L of drinking water)+Vitamin E (100 mg/kg orally)
Group 5: CdCl2 (10 mg/L of drinking water)+Zinc (15 mg/kg oral administration in drinking water)
Group 6: CdCl2 (10 mg/L)+Vitamin C (100 mg/kg BW)+Zinc (15 mg/kg)
Group 7: CdCl2 (10 mg/L)+Vitamin E (100 mg/kg)+Zinc (15 mg/kg)
Group 8: CdCl2 (10 mg/L)+Vitamin C (100 mg/kg BW)+Vitamin E (100 mg/kg)+Zinc (15 mg/kg)
Quantity of food consumed by rat; 35-60 g forage/day and of drinking water 25-40 mL/day.
After completing the study, all the animals were anesthetized, all the animals were anesthetized and blood samples were collected through cardiac puncture. Animals were sacrificed by cervical dislocation and the rats’ liver was removed. Serum samples were separated by using centrifugation at 2000 rpm for 20 min. Serum samples were used for biochemical analysis. The liver was weighed to their nearest mg using Shimadzu Electronic Balance and was used for experimental purposes.
Biochemical analyses were performed by following methods:
The organ weight was presented as relative organ was calculated as follows:
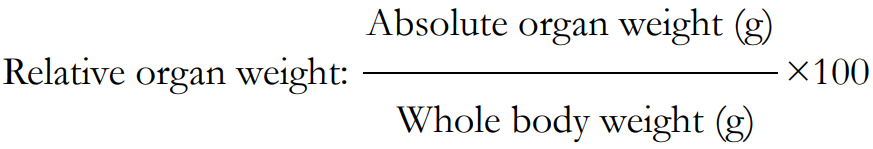
Aspartate aminotransferase (AST) (EC: 2.6.1.1): Reitman et al12
Alanine aminotransferase (ALT) (EC: 2.6.1.2): Reitman et al12
Alkaline phosphatase (ALP) (EC: 2.3.1.1): Rosalki et al13
γ-Glutamyl transpeptidase (GGT) (EC: 2.3.2.2): Novogrodsky et al14
Lactate dehydrogenase (LDH) (EC: 1.1.1.27): Kornberg15
Urea: Patten et al16
Creatine: Faulkner et al17
Protein: Lowry et al18
Glucose: Roe19
Statistical Analysis and Data Presentation
All the obtained data were statistically analyzed using SPSS package. Results obtained were presented as Mean±SD for comparison of different experimental animal groups with control ones. The results were statistically analyzed by a one way ANOVA. p value<0.05 was considered significant. Data of biochemical measurements were further subjected to estimation of percent of changes caused by exposure to the heavy metal CD and the improvement achieved by co-administration of Vitamin C, E and Zinc amelioration index (AI).
RESULTS AND DISCUSSION
In the present study eight groups of rats were maintained for a period of 45-days. No mortality occurred during the experimental period.
Body Weights and Relative Liver Weights
Final body weights and relative liver weights of male rats subjected to different experimental treatments were obtained and presented in Table 1. The Final body weights were significantly decreased -36.68% (p<0.05) with CD-treated rats compared to control and other co-treatment groups Vitamin C, E and Zinc with CD individually combinations with CD groups. The final body weights were 256.56 g in the control group, and 143.18 g with CD treated group. In contrast, co-treatments with Vitamin C, E and Zinc recorded the body weights 233-238 g range, but the co-treatments with combinations yielded 242-246 g. Among the treatments, all the groups recorded a weight gain (NS) except in CD treated group (p<0.05) which is significant. Ameliorative index calculated recorded to be 62.82, 66.47 and 65.64% with groups pretreated individually with Vitamin C, E and Zinc with CD, respectively compared to only CD treated group which is highly significant (p<0.05). But the combination of Vitamin C, E and Zinc with CD, the Ameliorative index was 69.09%, 70.03% and 72.35%, respectively and are significant (p<0.05) when compared to CD treated group of rats. The body weight gain was 31.28, 8.02, 14.16 and 11.84 g compared to the control group (p<0.05), and with pretreated Vitamin C, E and Zinc with CD groups individually, respectively which are statistically not significant (NS). In contrast, the body weight gain was 16.79, 17.27 and 20.98 g compared to the pretreatment groups of Vitamin C, E and Zinc, combined with CD treatment (p<0.05). But the CD treated group recorded a loss of 32.95 g during experimentation (p<0.05). Liver weights decreased in all experimental groups (p<0.05) with the CD treated group and pretreated Vitamin C, E and Zinc. The rate of change for the reported reductions in weight was not different from the pretreatment groups (Vitamin C, E and Zinc) combined with CD. The histo-somatic index (HSI) of the liver was approximately 3.0 for all groups.
| Table 1. Body and Relative Organ Weights of Control and Experimental Rats |
|
|
|
|
| Parameter |
Control |
Cadmium Treated |
Cadmium+Vit-C Treated |
Cadmium+Vit-E Treated |
Cadmium+Zinc
Treated
|
Cadmium+Vit-+Zinc Treated |
Cadmium+Vit-E+Zinc Treated |
Cadmium+Vit-E+C+Zinc Treated |
| Initial body
weight (g) |
225.28±10.04 |
226.13±10.59 |
225.11±9.14 |
224.19±9.74 |
225.82±8.54 |
225.32±8.49 |
226.18±8.44 |
225.79±8.48 |
| Final body weight (g) PDC |
256.56±10.95
+13.70b
|
193.18±10.32
+36.68aPDE |
233.13±2.04
+3.56c+62.82a
|
238.35±10.14
+6.32c+66.47a
|
237.16±10.05
+5.02c+65.64a |
242.11±10.13
+7.45c+69.09a
|
243.45±10.18
+7.64c+70.03a
|
246.77±10.73
+9.29c+72.35a
|
| Body weight gain/loss (g) in 45-days |
31.28±1.15 |
32.95±1.14 |
8.02±0.33 |
14.16±0.48 |
11.84±0.56 |
16.79±0.64 |
17.27±0.84 |
20.98±0.88 |
| Liver weight (g)PDC |
8.95±0.18 |
5.73±0.15
-35.98a |
7.36±0.16
-17.77b
|
7.39±0.17
-17.43b
|
7.42±0.17
-17.09b |
8.45±0.18
-5.59c
|
8.42±0.23
-5.92c
|
8.18±0.21-8.61c |
| HSI of liver |
3.49 |
2.97 |
3.16 |
3.10 |
3.13 |
3.49 |
3.46 |
3.31 |
| All Values are Mean±SD of six individual observations. Values are statistically significant at (a) p<0.05, (b) p<0.01. (c) NS: Not Significant. PDC: Percent Deviation over control group. PDE: Percent Deviation over Experimental group i.e. Cadmium treated group. HIS: Histo Somatic Index. |
Liver Dysfunction
To determine the extent of CD-related toxicity and the subsequent amelioration of the toxicity with Vitamin C, E or Zinc co-administration, we measured AST, ALT, GGT, LDH activities in plasma. Our results demonstrated that enzyme activity levels were significantly (p<0.05) increased by 76.24% following CD treatment compared to 64.31%, 68.44% and 66.78% with pretreated Vitamin C, E and Zinc, respectively. However, the percent change was relatively low yet significant (p<0.05) recorded as 48.32, 41.05 and 28.03% with pre-treated Vitamin C, E and Zinc in combinations with CD groups, respectively (Table 2). But ALP activity was found to be significantly (p<0.05) decreased in all the experimental groups. This reduction suggests that CD induces liver toxicity and that Vitamin C, E and Zinc were able to protect against this toxicity. The Creatine, Glucose and Urea contents were found to be significantly elevated (p<0.05) in all the experimental groups compared to control group.
| Table 2. Changes in the Serum Enzymes and Blood Parameters in Control and Experimental Rats |
| Parameter/ Serum |
Control |
Cadmium Treated |
Cadmium+Vit-C Treated |
Cadmium+Vit-E Treated |
Cadmium+Zinc Treated |
Cadmium+Vit-C+Zinc Treated |
Cadmium+Vit-E+Zinc Treated |
Cadmium+Vit-E+C+Zinc Treated |
| AST1 |
48.77±1.52PDC |
85.95±1.78
+76.24aPDE |
80.13±1.82
+64.31a-6.77c |
82.15±1.84
+68.44a-4.42c
|
81.34±1.79
+66.78a-5.36c
|
72.34±1.53
+48.32a-15.84b |
68.79±1.74
+41.05a-19.97b
|
62.44±1.29
+28.03a-27.35a
|
| ALT1 |
37.86±1.28PDC |
67.94±1.29
+79.45aPDE |
58.44±1.28
+54.36a-13.98b |
58.79±1.62
+55.28a-13.47b
|
59.12±1.49
+56.15a-12.98b
|
54.71±1.62
+44.51a-19.77b |
48.69±1.48
+28.61a-28.33a
|
45.73±1.54
+20.79a-32.69a
|
| ALP1 |
102.39±3.78PDC |
40.77±1.05
-60.18aPDE |
61.11±1.22
-40.32a+49.89a |
63.13±1.24
-38.34a+54.84a
|
64.31±1.39
-37.19a+57.74a
|
70.92±2.91
-30.74a+73.95a |
75.99±2.88
-25.78a+86.39a
|
80.42±2.75
-21.46a+97.25a
|
| GGT1 |
18.73±1.04PDC |
30.76±1.34
+64.23aPDE |
27.49±1.28
+46.77a-10.63b |
26.89±1.48
+43.57a-12.58b
|
27.13±1.39
+44.85a-11.80b
|
25.14±1.12
+34.22a-18.27b |
25.38±1.16
+35.51a-17.49b
|
22.15±1.19
+18.26a-27.99A
|
| LDH1 |
214.73±10.05PDC |
389.92±20.14
+81.59aPDE |
340.15±15.94
+58.41a-12.76b |
338.74±18.45
+57.75a-13.13b
|
342.05±20.10
+59.29a-12.28b
|
302.19±19.12
+40.73a-22.50a |
290.13±18.12
+35.11a-25.59a
|
278.77±16.79
+29.82a-28.51a
|
| Creatinine2 |
11.14±0.39PDC |
17.34±0.48
+55.66aPDE |
15.08±0.42
+35.37a-13.03b |
15.14±0.41
+35.91a-12.69b
|
15.32±0.42
+37.52a-11.65b
|
13.79±0.44
+23.79a-20.47a |
14.05±0.45
+26.12a-18.97a
|
13.34±0.42
+19.75a-23.07a
|
| Glucose3 |
0.87±0.05PDC |
1.73±0.12
+98.85aPDE |
1.44±0.14
+65.52a-16.76b |
1.38±0.13
+58.62a-20.23b
|
1.39±0.13
+59.77a-19.65b
|
1.22±0.14
+40.23a-29.48a |
1.28±0.15
+47.13a-26.01a
|
1.13±0.14
+29.89a-34.68a
|
| Urea2 |
0.53±0.03PDC |
0.88±0.05
+66.04aPDE |
0.74±0.05
+39.62a-15.91b |
0.76±0.05
+43.40a-13.64b
|
0.78±0.06
+47.17a-11.36b
|
0.68±0.06
+28.30a-22.73a |
0.69±0.08
+30.19a-21.59a
|
0.64±0.08
+20.75a-27.27a
|
| All Values are Mean ±SD of six individual observations. Values are statistically significant at (a) p<0.05, (b) p<0.01. (c) NS: Not Significant.. PDC: Percent Deviation over control group. PDE: Percent Deviation over Experimental group, i.e. Cadmium treated group. AST: Aspertate Amino transferase; ALT: Alanine Amino transferase. ALP: Alkaline Phosphatase; GGT: Gamma-glutamyl transferase, LDH: Lactate dehydrogenase 1: IU/ml/hr; 2: g/lit; 3: mg/lit, 1,b Means in the same column not followed by the same letter differ significantly p<0.05 |
In the present study, an attempt was made to evaluate CD’s toxic effects in rats; furthermore, we are very interested in knowing whether Vitamin C, E and Zinc as an antioxidant can recover the toxic effects caused by CD. CD has been recognized as one of the most toxic environmental and industrial pollutants and has been reported to induce oxidative damage. Elevated oxidative stress is due to the disruption of the prooxidant-antioxidant balance in the tissues. Earlier reports suggest that heavy metals manifest their toxic effects by enhanced production of reactive oxygen species (ROS) production, a major cellular source of oxidative stress.1 ROS can damage every major cellular component, including membrane lipids, carbohydrates and deoxyribonucleic acid (DNA). The pathological consequence of such uncontrolled is wide spread tissue damage.1 This present study’s main objective was to evaluate the biochemical and pathological changes in following CD treatment in Wistar rats and the ability of Vitamin C, E, and Zinc to attenuate CD-mediated toxicity alone and in combination.
Body Weight and Relative Liver Weight
In toxicological studies, it has been reported that body and organ weights are considered as an important criterion for evaluating organ toxicity. The increase or decrease in body weights is a sign of toxic effects of xenobiotics. Body weight and relative liver weights of rats treated with CD or pretreated with Vitamins C, E or Zinc, either individually or in combination, followed by CD treatment, were significantly reduced. Immunization causes pain, distress, and inflammation, which reduces the animal’s movement and appetite, thereby significantly decreasing food intake (anorexia or food avoidance) or poor food palatability due to CD treatment. CD-mediated toxicity involves the induction of oxidative stress resulting in alterations in the antioxidant status, leading to metabolic disorders and weight loss. Several authors reported that inflammation causes weight loss between 1-20% during CD exposure. Our study investigated Vitamin C, E, and Zinc’s potential to attenuate CD-mediated toxicity and restore CD-treated rats’ metabolic status, increasing food intake, body weight, and liver/body weight ratios. There was a significant (p<0.05) increase in body weight in the antioxidant-treated groups across all groups compared to the CD-treated animals.
Liver Dysfunction
From the results obtained, CD exposure clearly induces the damage that occurred in liver and other tissues as observed through pathological studies. The liver and kidney are important organs for metabolism, detoxification, storage and excretion of xenobiotics and their metabolites. The physiological function assigned to the liver and kidney suggests they are especially vulnerable to damage. As the liver is an important target organ for xenobiotic, we have also assessed the liver and its associated functions for CD-induced toxicity. Serum enzymes including ALP, ALT, AST, GGT and LDH are mainly considered as biomarkers for the evaluating of hepatic damage due to xenobiotic treatment. In the present study, following CD-exposure, serum AST, ALT, GGT and LDH were significantly elevated. In contrast, ALP activity showed a significant decrease in the activity levels than normal or control rats. In addition, increased levels of hepatic serum markers suggest an extensive liver injury. Lipid peroxidation is one of the main manifestations of oxidative damage, which always plays an important role in the toxicity of many xenobiotics.2,20,21
The data obtained in the present study also confirm that CD-intoxication causes a significant increase of lipid peroxidation concentration in liver tissue of rats. Since it causes lipid peroxidation in numerous tissues both in vivo and in vitro,22 CD may induce oxidative stress by production of hydroxyl radicals,23 superoxide anions, nitric oxide and hydrogen peroxide.24,25 Cadmium exposure causes structural and functional damages to the cell membrane, significantly increasing permeability resulting in the leakage of hepatic enzymes into the blood. Furthermore, the liver damage due to oral administration of CD chloride was confirmed through the increase in the levels of plasma components, including bilirubin. Therefore, increased in the activities of AST and ALT activities in plasma is mainly attributed to the leakage of these enzymes from the liver cytosol into the blood stream. Reports are available that lysosomal instability caused by CdCl2 resulted from leakage of hepatic enzymes including ALT, AST and ALP into the blood stream.26 In the present study, a significant (p<0.05) increased AST activities; ALT changes may be attributed to the hepatic damage due to CD intoxication. Meanwhile, the alteration of serum ALP levels may also be attributed to cholestasis and acute hepatocellular necrosis. Several authors reported that due to CD treatment, the liver enzymes like SGOT, SGPT and ALP were significantly elevated compared to control group of rats, denoting liver dysfunction. Serum transaminases represented by AST and ALT were significantly elevated during CD-intoxication, indicating the loss of cellular integrity and the leakage of hepatic membrane. In the present study, the hepatocellular injury was associated with CD intoxication observed (Unpublished data).
El-Kady et al,27 found that rats treated with CD (CD2+) ions alone showed a significant increase in serum enzymatic activities such as ALT and AST activities accompanied by significant decrease in the total protein content. Our results demonstrate that the marked changes in liver enzyme activities represent biomarkers for liver damage. Genchi et al3 found that the increase in activities of these enzymes, including AST, ALT and ALP in serum after CD treatment, reflect the destructive effect of CD on cell membrane, resulting in increased release of functional enzymes from intracellular locations, which indicates the hepatotoxic effect of CD. Chavan et al28 reported that serum GPT, ALP, bilirubin, blood urea were significantly increased in response to CD intoxication, indicating liver function impairment to an increase of CD-induced oxidative stress. Serum LDH activity also increased significantly due to the hepatocellular necrosis leading to leakage of enzyme into the blood stream. It has been previously reported that during liver damage, there was an observed decrease in anti-oxidant defenses in the liver.3 The hepatic function test corroborated the histopathological lesions observed (Unpublished data). These observations indicated that marker enzymatic activity changes followed by the liver’s overall histoarchitecture in response to CD-toxicity, which could be due to its toxic effects primarily by the generation of ROS causing a significant damage to the various membrane components of the cell. The prevention of lipid peroxidation is essential for all aerobic organisms so the organism is well-equipped with antioxidants that protect cells against the adverse effects of various toxicants.29 Antioxidants’ role in reversing the oxidative stress-induced due to xenobiotic intoxication has been of long-standing interest to basic scientists and clinicians.29 The results obtained in the present study demonstrate that co-administration of Vitamin C, E and Zinc either individually or in combinations with CD reverted most of these altered biochemical parameter levels to within normal limits and substantially improved liver function. A partial amelioration of this damage by Vitamin C, E and Zinc would be attributed to antioxygenic role and Vitamin E as a free radical scavenger and an effective inhibitor of autocatalytic process of lipid peroxidation. Several authors reported that Vitamin E is the most important lipophilic antioxidant and exists mainly in the cellular membranes, thus helping to maintain the membrane stability.9,30
Cadmium exposure results in hyperglycemia and is due to the toxic action of this metal on the secretory activity of the pancreas. The elevation of serum glucose in the present study due to CD intoxication may be attributed to inhibition of insulin release from islets of langerhans.31,32 Inhibition of glucose uptake in the target tissue or resistance to insulin action has also been reported.33,34 Disruption in glucagon secretion results in high glycogen breakdown and a new supply of glucose production from other non-carbohydrate sources such as proteins.35 However, there is an amelioration of blood glucose concentration in CD-treated animals with Vitamin C, E and Zinc, alone or in combination.
Consequently, treatment with Vitamin C, E and Zinc will improve glucose concentration in CD-treated Wistar rats. Improvement of the glucose status suggests that Vitamin C, E and Zinc supplementation will cause a decrease in CD effect in salt binding to biomolecules as well as improved insulin secretion by reducing glucose accumulation. Zinc protects enzymes, and ATP involved glucose metabolism. During tissue toxicity, including CD-induced toxicity, free radicals generated by oxidative damage to membrane lipids and lipoproteins can cause cellular damage and apoptosis.20,37 Oxidative damage to cell membranes can further culminate into pathological changes in the histomorphology of exposed tissues. However, Vitamin C and E, natural antioxidants, can potently cause inhibition or scavenging of free radicals, thereby lessening the damage effects of tissue toxicants. It is an essential and Vitamin C and anti-toxin which potently functions to reduces the harmful effects of toxic agents in biological tissues. Each molecule of ascorbic acid contains two hydrogen atoms that bear two high-energy electrons, which can be readily donated to reduce oxidation by free radicals, thereby neutralizing or alleviating the harmful effects of tissue toxins.38
Increased serum urea and creatinine levels in the CD-treated rat group may be attributed to an oxidative imbalance in the kidney, leading to elevated urea and creatinine in the blood and correlate with the earlier reports,39 where urea level was elevated as a result of CD-intoxication. The significantly (p<0.05) elevated level of creatinine in the CD-intoxicated rat group may be attributed to the oxidative damage to the kidney. Deterioration of the kidney would permit creatinine release into the blood and agree with earlier reports, where CD administration leads to an elevated level of creatinine.40 Kidney damage was also linked to the defect in infiltration. Shaffi41 also reported that rise in creatinine level is an indication of renal-tubular damage due to CD-induced nephrotoxicity. The reduction of the urea and creatinine level by CD may suggest that Vitamin C, E and Zinc exerts hepato- and nephro protective effects when exposed to CD.
CONCLUSION
Based on the present study results, it could be evident that CD has a harmful and stressful effect on hepatic, renal and hematological tissues. However, either individually or in combinations Vitamin C, E and Zinc had protective effects against CD-induced oxidative damage or stress. Also, from our results, we conclude that Vitamin C, E and Zinc have potent antioxidant activity against CD toxicity. The consumption of foods rich in vitamin C & E is recommended to reduce the damage caused by CD’s toxicity. Hence, Vitamin C, E and Zinc can be regarded as good therapeutic agents against CD toxicity.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.