INTRODUCTION
Pyrethroids are the second widely used insecticides to control agricultural and indoor pests.1 Due to wide usage, pyrethroids have been detected in non-target organisms, including fish and human.2 Cypermethrin, a member of the family of synthetic pyrethroids of type II class, is extensively used in agricultural and other domestic applications. It is well-established that cypermethrin, both cis- and trans-isomers are metabolized to phenoxybenzoic acid and cyclopropane carboxylic acid.3 Populations at the highest risk of high-dose exposure are producers, hygienic, and pesticide workers, and small farm owners applying cypermethrin for plant protection. Low-dose exposure originates mainly from the household application of insecticides, contaminated food, and water.4
There are substantial evidences that pyrethroids create toxicities apart from their actions in the nervous system.5,6 Immune-insufficiency of allethrin, cypermethrin, fenpropathrin, permethrin was studied with regard to pyrethroid insecticides.7 The immune system comprising specialized, memorized complex cells, tissues and organs and they exhibit innate and adaptive responses to protect organisms from different pathogens as well as to maintaining life processes. The relevant interaction between immune response alteration and stress are shown by various epidemiological and experimental studies.8 Synthetic pyrethroid might induce stress-like symptoms in experimental animals.9 In immunotoxicological studies with synthetic pyrethroid insecticide cypermethrin, dose dependent suppression of humoral and cell-mediated immune response was induced.10 It has been reported that pyrethroid insecticides are genotoxic in mouse spleen and bone marrow as well as in cultured mouse spleen cells.11
As important trace element zinc controls immune function and cell proliferation.12,13 Through metallothionein zinc potentiates antioxidant system that impedes oxidative stress facilitated cell injury.14,15
By assisting in acyl-group transfer alpha (α)-lipoic acid play role as a coenzyme in the TCA cycle and is considered as a food supplement exhibiting its antioxidant properties. Through the reduction of free radicals it safeguards diabetes mellitus, aging, neurodegenerative and vascular diseases.16,17,18
As the immunotoxicity of synthetic pyrethroid cypermethrin is not well-explored, we have focused our present study on the humoral and cellular immune responses of cypermethrin in a rat model.
According to Goel et al,19 pretreatment of zinc to chlorpyrifos intoxicated animals significantly improved the blood toxicity (at the dose level of 227 mg/L in drinking water). Andreeva-Gateva et al,20 evaluated the effect of alpha-lipoic acid (35 mg/kg i.p.) on brain oxidative stress (OS) in unilateral intrastriatal (6-OHDA) injected rats.
The prophylactic effect co-administration of coenzyme Q10 and alpha-lipoic acid was reported in experimentally cisplatin-induced nephrotoxicity of male albino rats.21 Co-administration of alpha-lipoic acid and vitamin E protect renal cells from injury caused by ROS mediated oxidative stress and related vascular complications induced by nano zinc oxide in rats.22
This study also aimed to explore the possible protective role of co-administered zinc and alpha-lipoic acid on any attenuation in immunotoxicity, if any, after oral exposure to cypermethrin in male albino rat.
MATERIALS AND METHODS
Chemicals
Cypermethrin 10% emulsifiable concentrate (EC) named ‘‘Ustad’’ (United Phosphorus Limited, Mumbai, India), Zinc sulphate (ZnSO4), white blood cell (WBC) dilution fluid, chloroform, ethylene-diamine-tetra-acetic acid, phosphate buffer, histopacque-1077, RPMI-1640 and other chemicals were procured from Merck Ltd., Himedia, Mumbai, India.
Animal Maintenance
Male Wistar albino rats weighing 130-150 g were selected for the study. Animals were acclimatized for 10-days before the experiment schedule. Rats were provided standard diet and water sufficiently. They were maintained under 25±2 °C (approximate) temperature and 12 h light-dark cycles throughout the period of experiment. Experimental protocol and surgical methods were reviewed and approved by the Institutional Animal Ethical Committee (IAEC), registered under Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA, New Delhi, India).
Treatment Protocol
Thirty-six male Wistar albino rats weighing 130-150 g were randomly assigned to the five experimental groups and one control group, each containing six rats. Groups were designed as: Group I: Control (5 mL/kg body weight), Group II: Zinc (227 mg/L in drinking water) and lipoic acid (35 mg/kg body weight) control, Group III: Cypermethrin-treated (Low dose, 40 mg/kg body weight), Group IV: Zinc and lipoic acid+Cypermethrin-treated (Low dose, 40 mg/kg body weight), Group V: Cypermethrin-treated (High dose, 80 mg/kg body weight), Group VI: Zinc and lipoic acid+Cypermethrin-treated (High dose, 80 mg/kg body weight) group.
Rats were treated orally following the outlined schedule each day at the same time for 14 consecutive days as described in OECD guideline.
On the 15th day, rats were sacrificed, blood samples and the specified internal organ (spleen) from control and treated rats were immediately collected for biochemical, hormonal and histological analysis.
Blood Collection
Blood samples were collected from the treated groups by cardiac puncture. Collected blood was allowed to pour take drop-by-drop into a graduated centrifuge tubes containing ethylene-diamine-tetra-acetic acid (EDTA) for the determination of total leukocyte count (TLC) and differential leukocyte count (DLC).
Total Leukocyte Count
Total leukocyte count23 was estimated by diluting blood in 1:20 dilution with WBC dilution fluid and total leukocytes were counted in a Neubaur haemocytometer chamber.
Differential Leukocyte Counts
Thin blood smear in a clean glass slide was stained with Leishman’s stain and it was observed under the microscope. The percentage of granulocytes and agranulocytes was calculated.23
Collection of Peritoneal Macrophages
The peritoneal macrophages were collected from the animals using i.p. injection of 5 ml of BSA with 5 cold PBS. After 24 h, cells were collected from peritoneal cavity and centrifuged at 1000 rpm for 10 min and it was suspended in RPMI 1640 medium containing 10% fetal bovine serum. Then the macrophage suspension was added to 96-well tissue culture micro-plates microplates at density 1×106 cells/well.
Study of Phagocytic Activity
Five hundred (500) μl of the aliquot of cells containing peritoneal macrophages (density of 2×106 cells/ml) was mixed with 500 μl of Roswell Park Memorial Institute (RPMI)-1640 containing 10% fetal bovine serum (FBS) and prepared charcoal solution. The mixture was applied to a glass slide and incubated for 2 h at 37 °C in a humid chamber. The phagocytic index was determined by checking the Giemsa-stained phagocytic cells under the light microscope.24
Serum Nitric Oxide (NO) Activity
The serum was suspended in phosphate buffered saline (PBS) and it was centrifuged at 10,000 rpm for 15 min. Then the cell-free supernatant was collected and nitric oxide released was measured using the Griess reaction.25
Determination of Total Immunoglobulin Concentration
Total serum immunoglobulin was determined by zinc sulfate turbidity test.26 Briefly, 25 µl of the collected serum were mixed with 1700 μl of 0.7 mM zinc sulfate at pH 5.8. The mixture was shaken and left for 1-hour at room temperature. Serum mixed with PBS at the same ratio was utilized as the blank or control. Optical density was measured spectrophotometrically at 545 nm wavelength.
Quantitative Hemolysis Assay
Quantitative hemolysis assay was done using the method of Simpson and Gozzo with some modifications.27 One (1) ml of serum was collected and incubated for 3 h at 37 °C. After centrifugation at 3000 rpm for 3 min, the optical density of the supernatants was determined at 560 nm using a spectrophotometer.
Proliferation Assay of Blood Mononuclear Cells
Blood mononuclear cells (BMCs) were suspended in RPMI 1640 medium supplemented with 10% FBS, 100 IU/mL penicillin and 100 μg/mL streptomycin. BMCs (5×105) from treated and control animals were cultured for 24 h. After 3 h of incubation at 37 °C in 5% CO2, the optical density was measured at 450 nm. The proliferation percentage was calculated by dividing each value (tested) by the average mean of the control samples multiplied by 100.26
Histological Examination of Spleen
The collected tissues from sacrificed animals were dehydrated in increasing ethanol concentrations, cleared with xylene and embedded in paraffin. Then 5 µ thick tissue sections were cut using microtome and stained with hematoxylin and eosin stain (H&E). Images of the histological sections were analyzed using light microscopy.
Statistical Analysis
The results were expressed as the Mean±Standard error of mean (SEM). Statistical analysis of the collected data was performed by analysis of variance (ANOVA) followed by two-tail t-test. The difference was considered significant when p<0.05.
RESULTS
Effect of Cypermethrin on Rat Leukocytes
Table 1 presented that the total WBC count and percentage of lymphocyte were significantly increased (p<0.001) as well as neutrophil % (p<0.001) were significantly reduced in low- and high- dose of cypermethrin treated rats compared to control group rats. zinc and lipoic acid co-administration ameliorate the total WBC count and neutrophil percentage (p<0.001).
| Table 1. Effect of Zinc and α-lipoic Acid on Total and Differential Leukocyte Count of Cypermethrin-Treated Rat |
|
WBC Count /µL |
Lymphocyte Count (%) |
Neutrophil Count (%) |
| Control |
5591±58 |
54.33±0.57 |
35.5±0.428 |
| Zinc and lipoic acid control |
5600±64 |
54 ±0.577 |
35.5±0.428 |
| Cypermethrin low dose |
7275±83a*** |
63.66±0.666a*** |
25.5±0.428a*** |
| Cypermethrin low dose+zinc and lipoic acid |
5716±83b*** |
53.66±0.666b*** |
35.5±0.428b*** |
| Cypermethrin high dose |
6866±102a*** |
64.833±0.477a*** |
23.6±0.494a*** |
| Cypermethrin high dose+zinc and lipoic acid |
5508±58c*** |
56.5±0.428a*c*** |
29.5±0.428a*c*** |
| Results are expressed as Mean±SEM. Analysis is done by ANOVA followed by multiple comparison two-tail t-tests. Superscript a, Group-I versus all other groups; (* indicates p<0.01; *** indicates p<0.001) |
Effect of Cypermethrin on Phagocytic Index
Figure 1 showed that phagocytic index (macrophage) in both low- and high- dose cypermethrin treated rats were significantly diminished (p<0.001) in comparison to the control group animals and pre-treatment with Zinc and lipoic acid significantly (p<0.001) increased the phagocytic index of macrophages.
Figure 1. Illustrates the Effect of Zinc and α-lipoic Acid on Macrophage Phagocytic Index in Cypermethrin Induced Male Albino Rat
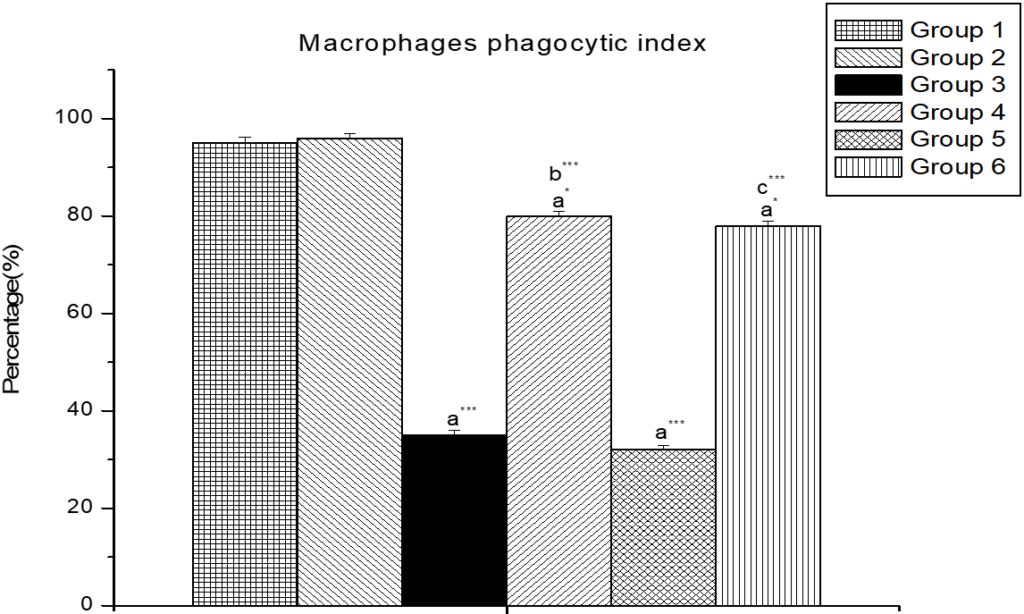
Results are expressed as Mean±SEM. Analysis is done by ANOVA followed by multiple comparison two-tail t-tests. Superscript a, Group-I versus all other groups; Superscript b Group-III vs Group-IV; Superscript c Group-V vs Group-VI. Asterisks represents the different level of significance (** indicates p<0.01;*** indicates p<0.001)
Impact of Cypermethrin on Nitric Oxide Activity
Pre-treatment of zinc and lipoic acid resulted in a significant decrease (p<0.001) in serum nitric oxide activity which were significantly increased (p<0.001) in the low- and high- dose of cypermethrin treated animals compared to control groups rat (Figure 2).
Figure 2. The Effect of Zinc and α-lipoic Acid on Serum Nitric Oxide Activity in Cypermethrin Induced Male Albino Rat
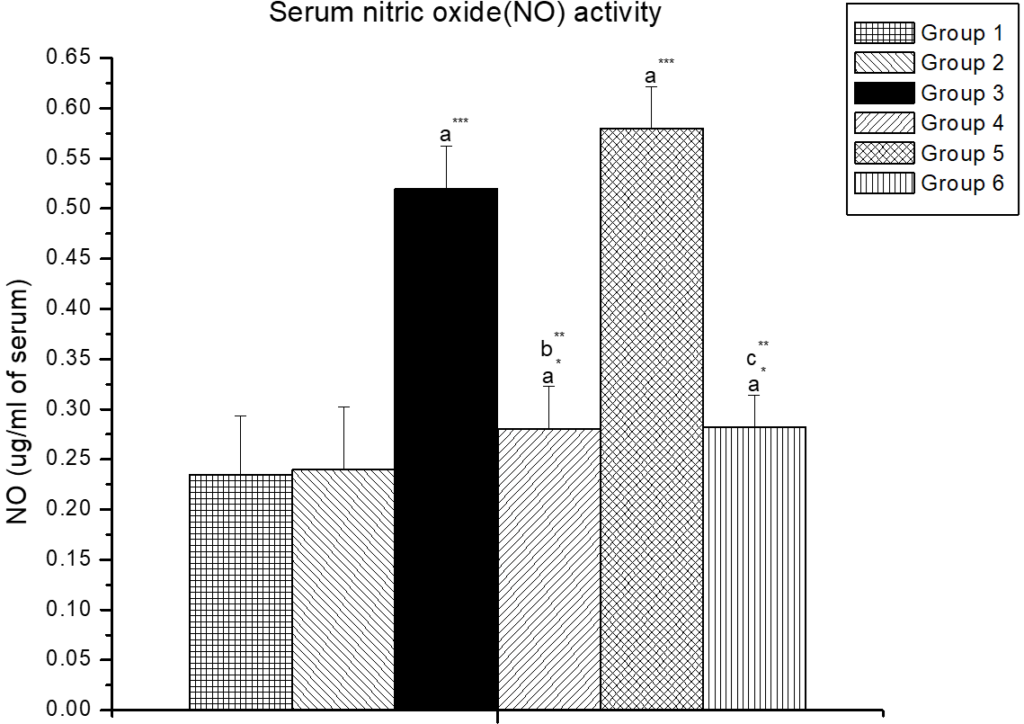
Results are expressed as Mean±SEM. Analysis is done by ANOVA followed by multiple comparison two-tail t-tests. Superscript a, Group-I vs all other groups; Superscript b Group-III vs Group-IV; Superscript c Group-V vs Group-VI. Asterisks represents the different level of significance (** indicates p<0.01;*** indicates p<0.001)
Effect of Cypermethrin on Total Immunoglobulin, Quantitative Hemolysis, Blood Mononuclear Cell Proliferation
As shown in Table 2, cypermethrin reduced total immunoglobulin concentration and blood mononuclear cell proliferation (p<0.001) whereas quantitative hemolysis was significantly increased (p<0.001) in cypermethrin treated rats and co-administration of zinc and lipoic acid returned it towards normal levels.
| Table 2. Effect of Zinc and α-lipoic Acid on Total Immunoglobulin conc. (g/L), Quantitative Hemolysis (%) and BMC Proliferation (%) of Cypermethrin-Treated Male Albino Rat |
|
Total Immunoglobulin con (g/L) |
Quantitative Hemolysis (%) |
BMC Proliferation (%) |
| Control |
5.12±0.21 |
0.134±0.001 |
85±1.5 |
| Zinc and lipoic acid control |
5.02±0.25 |
0.136±0.002 |
82±2 |
| Cypermethrin low dose |
3.02±0.22 a*** |
0.189±0.002a ** |
50±1.5a** |
| Cypermethrin low dose+zinc and lipoic acid |
4.48±0.12 a*b*** |
0.131±0.002b** |
72±1.2a*b** |
| Cypermethrin high dose |
3±0.23a*** |
0.198±0.003a** |
38±1.2a*** |
| Cypermethrin high dose+zinc and lipoic acid |
4.52±0.24 a*c*** |
0.135±0.001c** |
65±1.5a*c** |
Results are expressed as Mean±SEM. Analysis is done by ANOVA followed by multiple comparison two-tail t-tests. Superscript a, Group-I versus all other groups;
(* indicates p<0.01; *** indicates p<0.001 |
Effect of Cypermethrin on Splenic Histology
Figure 3 showed marked alteration in splenic red and white pulp area in low and high dose cypermethrin treated animals. In zinc and lipoic acid pretreated rats, the architecture of the spleen was altered towards normal.
Figure 3. The Effect of Zinc And α- Lipoic Acid on Splenic Histology of Cypermethrin Induced Male Albino Rat
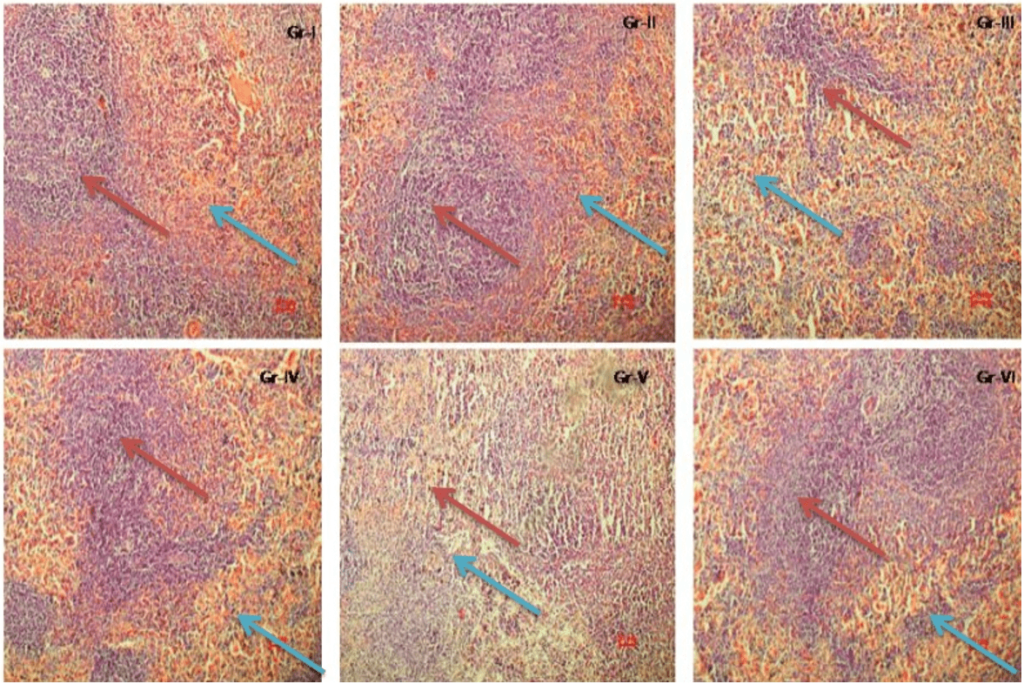
Red arrow indicates red pulp; blue arrow indicates white pulp. Group I shows normal red pulp and white pulp also group II shows normal red pulp and white pulp. Group III shows disintegration of red pulp and white pulp. In Group IV we find out the normalization of white pulp and red pulp. But in Group V red pulp and white pulp are not clear. But Zinc and alpha lipoic acid has tried to normalize the histology. Bar scale 10 µm. Magnification 200X.
DISCUSSION
The acute toxicity of many pesticides is well-known and poisoning cases are often reported. In contrast, much less is known about long-term impacts on human or different animal systems including the nervous, hormone, reproductive and immune systems. Immunotoxicity of pyrethroids have been reported earlier by several researchers.28,29 Immunosuppressive effects associated with high doses of deltamethr in28 and fenvalerate30 on humoral and cell-mediated immune responses in different species like adult mice, rats, and goats have been reported.
The present study may be considered as a part of sub-acute toxicity study and reports the alterations of immunological parameters and the beneficial effects of zinc and lipoic acid treatment in cypermethrin intoxicated male rats.
The increased leukocyte (WBC) counts were noted in cypermethrin treated groups and it may be due to the activation of the defense and immune systems of the body.31 This may results in an increased release of WBC from the bone marrow storage pool into the blood. The primary function of white blood cells is to defend against foreign bodies, which is attained by leucocytosis and antibody production. Pathological leucocytosis may occur due to exposure of chemicals or acute haemorrhages and haemolysis. Leucocytosis may be raised due to resistance of the animal for localization of the inflammatory response. Another possible cause of leucocytosis may be the severe haemorrhages in liver and lungs.32 This increase may be related to an increase in lymphocyte percentage. These results indicated that zinc and lipoic acid might have a beneficial role in lowering pyrethroidstoxicity probably due to its radical scavenging property.33
On the contrary, neutrophil counts were found to be decreased after 14-days of cypermethrin treatment. Neutrophils act as defence cells against foreign materials34 It is usually recognized that the innate immunity actions of neutrophils are generally facilitated by phagocytosis, discharge ofgranules, and development of neutrophil extracellular traps (NETs).34 It may be due to decreased immunity for the increased cypermethrin intoxication. Simultaneous co-administration of zinc and lipoic acid to cypermethrin treated animals improved the altered levels of haematological parameters. Interestingly, zinc and lipoic acid pre-treatment to cypermethrin intoxicated rats restored the levels of total as well as differential WBC count to the normal levels. These observations might also indicate that zinc and lipoic acid have therapeutic and beneficial effects on cypermethrin-mediated toxicity.
Phagocytes detect infected microbial pathogens and activate innate immune responses and it is crucial for maintaining or restoring host homeostasis.35 Macrophages as a phagocyte have key roles in host defense against microbes mediated infections by eliminating the pathogens producing modulation of immune responses. Both macrophages and neutrophils are conscripted to the inflamed site from circulating blood during microbial infection to notice, exterminate, and engulf the invading microorganisms. Significant inhibition in humoral immune response was detected in animals treated with high- and low- dose of cypermethrin. The detected reduction of humoral immune response assured the immunosuppression demonstrated after exposure to type II pyrethroids in the murine model. Nonspecific cellular immune response that examined in the current study showed significant inhibition in the phagocytic activity of peritoneal macrophages in cypermethrin-treated rats as compared to the control animals. Zinc and lipoic acid pretreatment ameliorate the toxicity.
Increased NO level designates adiminished antioxidative defence mechanism in arsenic-NaAsO2-induced immunotoxicity in vivo.36 To investigate the changes of immune status, we measured the levels of nitric oxide in the serum. As shown in Figure 2, exposure to cypermethrin was related to an increase in the level of nitric oxide level compared to the control group. Co-administration of zinc and alpha lipoic acid significantly decreased the level of nitric oxide in the serum compared with animals treated with cypermethrin alone.
In the present study a decrease in total immunoglobulin concentration in cypermethrin treated rat was detected. Previous findings indicated that most of type II pyrethroids (cypermethrin, super-cypermethrin forte, fenvalerate, deltamethrin and lambda-cyhalothrin) are known to cause impairment and suppression of immune system in adult rats and rabbits.10,37 In the cypermethrin treated groups, a significant reduction in total immunoglobulins concentration was detected, which may indicate diminished B-lymphocyte function with the resultant decreased antibody production.38
The histopathological findings of the present study revealed that the presence of immunotoxic effects in cypermethrin treated animals. The noticeable alteration in red and white pulp area was observed in the cypermethrin treated rat spleen. The congestion witnessed in the spleen may be as a result of intrasplenic damage of erythrocytes.39 These findings are in agreement with previous studies39 where exposure of permethrin adversely affected the function of spleen and thymus, important immune organs.
Zinc influences multiple facets of the immune system.40 Zinc is essential for maintenance of macrophages, neutrophils, and NK cells and development of cells mediated innate immunity. Zinc deficiency severely affects phagocytosis, intracellular killing, and cytokine production as well as the growth and function of T- and B-cells. Zinc as an antioxidant stabilizes membranes possibly by preventing free radical-mediated inflammatory processes.
Alpha lipoic acid, a natural ingredient of human body, not only acts as a powerful antioxidant but also is able to regulate the immune system in either direct or indirect ways.41 ALA is used to treat autoimmune diseases including systemic lupus erythematosus, rheumatoid arthritis, and primary vasculitis.
Co-administration of zinc and alpha lipoic acid significantly attenuated cypermethrin induced immunotoxicity in male Wistar rat probably for their above said properties.
CONCLUSION
Thus, from the findings we may conclude that cypermethrin caused prominent alterations in haematological parameters as well as immunotoxicity in male Wistar rat by impairing the immune status of the body. From the above findings, it is evident that zinc and alpha-lipoic acid have potent ameliorative role on cypermethrin induced immunotoxicity due to its antioxidant and immune status protecting properties.
ACKNOWLEDGEMENT
The authors are thankful to the authority of Vidyasagar University, Midnapore, India for providing all the facilities to execute this study.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.








