INTRODUCTION
Tramadol was launched under the name Tramal in 1977 by the West German pharmaceutical company Grünenthal GmbH.1 It is an opioid pain medication used primarily to treat mild to severe pain, both acute and chronic.2,3 When taken by mouth in an immediate-release formulation, pain relief usually begins within an hour. Tramadol is also available by injection or in combination with paracetamol (acetaminophen) or as longer acting formulations.2
The pharmacological influence of tramadol is elicited by binding to the μ-opioid receptor of the neuron and is also both serotonin and norepinephrine reuptake inhibitor.1 Tramadol is converted in the liver to O-desmethyltramadol; an opioid with stronger binding affinity for the μ-opioid receptor.4 The most common adverse effects of tramadol include nausea, dizziness, dry mouth, indigestion, abdominal pain, vertigo, vomiting, constipation, drowsiness, and headache.5,6 Compared to other opioids, respiratory depression and constipation are considered less of a problem with tramadol.6
Recognized risk factors for tramadol overdose include depression, addiction, and seizures.7 Deaths with tramadol overdose have been reported and are increasing in frequency in Northern Ireland; the majority of these overdoses involve other drugs including alcohol.7 There were 254 tramadol-related deaths in England and Wales in 2013, and 379 in Florida in 2011.8 In 2011, 21,649 emergency room visits in the United States were related to tramadol.9 The statistics of tramadol related morbidity and mortality is not available in Nigeria. However, the heavy reliance on tramadol resulted in the Government of the Federal Republic of Nigeria issuing a manufacturing ban in late 2018, prohibiting the manufacture, importation and sales of tramadol and its analog.10 This was done as a result of the increase in crime rates which was attributed to tramadol abuse.
Full blood count is a laboratory investigation that numerically estimates the various blood cell types suspended in the plasma. The cell types include red blood cells, white blood cells, and platelets. Holistically, full blood counts encapsulate the condition of the various cell types, the number and concentration. These indices are altered in course of diseases and toxicities. Furthermore, full blood count also provide important information about the blood and blood-forming tissues (especially the bone marrow and the liver for infants), as well as other body systems. Abnormal results are indicative of a variety of ill conditions such as aneamias, leukemias, and infections. Poisoning also distort the physiological balance of blood cells and plasma volume.
Red cell indices most frequently used in the investigation of diseases and toxicity are mean cell haemoglobin concentration (MCHC), mean cell volume (MCV) and mean cell haemoglobin (MCH). Red cell indices are crucial in the classification of anaemia and differential diagnosis of arrays of diseases.11 Red cell indices are a part of full blood count (FBC) or complete blood count (CBC), which reveals the complete status of various blood components in the individual’s. The values of MCV, MCH, and MCHC are derived from the values of haemoglobin, packed cell volume (PCV) or haematocrit and red cell count. Alterations of red cell indices are indicative of wide range of diseases.
The medical and societal effects of tramadol are incalculable due to the acute and chronic damages inflicted on the body. The entire body sustenance is a product of well-coordinated haematological processes. A deviation is an orifice to a lot of idiopathic and non-idiopathic diseases. The effect of tramadol on haematological profiles has been studied with diverging perspectives, though with different study animals and designs.12,13,14,15 Most of the studies did not consider the acute and chronic intoxication perspective either individually or holistically. This study is therefore crafted to evaluate the true presentation of haematological parameters upon exposure to either acute or chronic tramadol intoxication in rats. The alterations observed in rats could create an inroad for a further studies to the validate the potency of acute and chronic tramadol intoxication on blood cells and red cell indices using human model.
MATERIALS AND METHODS
Study Area
The animal breading and inducement were carried out using the Pharmacology Laboratory of the Niger Delta University (NDU), Wilberforce Island, Amasoma, Bayelsa State. Similarly, the laboratory investigations took place at the Haematology Laboratory of the Federal Medical Centre Yenagoa, Bayelsa State and the Niger Delta University Teaching Hospital, Okolobiri, Bayelsa State respectively. Bayelsa state is a state in Nigeria located within Latitude 40° 151 ′ North and Latitude 50° 231 South, longitude 50° 221 West and 60 451 East.16
Study Population
The study utilized Mead’s Resource Equation for the sample size determination.17 The study was categorized into acute and chronic phases. The acute phase was further divided into two groups; controls and tramadol intoxication death. The chronic phase comprised of four graded groups of different concentrations of tramadol intoxications. Each group constituted of six rats paired with a weight and age matched-controls. In total sixty rats were used for the entire study. The acute phase was concluded within twenty-four-hours, whereas the chronic took three-months, excluding two-weeks of acclimatization.
Ethical Approval
The ethical clearance and experimental protocol were approved by the Ethics Committee of the Bayelsa State Ministry of Health. The animals used for the study were humanely treated in accordance with the Animal Welfare Act of 1985 of the United States of America for Research and Institutional Animal Care and Use Committee (IACUC) protocols as posited by Benjamin et al.18
Selection Criteria
Albino rats used were healthy and active as confirmed and approved by a veterinarian. Rats showing signs and symptoms of illness or deformities were excluded from the research. The research utilized only male albino rats of same age and weight. The research utilized only male albino rats of same age (6-8-months) and weight (280-340 g). Also, lysed and contaminated samples were rejected.
Collection of Sample
Blood samples were collected from the heart using the method described by Ness.19 The samples were withdrawn slowly from the heart into EDTA containers for haematological investigations. Haematological investigations carried out include full blood count (FBC), red cell indices (RCI) and film reading.
Laboratory Procedures
Full blood count and red cell indices were estimated in whole blood sample using automated analyzer. The brand of automated analyzer used was SYSMEX Automated Blood Count machine (SYSMEX KX-21N ANALYZER). The film reading was performed using a compound microscope (Olumpus).
Statistical Analysis
The haematological data obtained were analyzed using student t-test and One-way ANOVA. Box-plots were also used for data presentation. JMP statistical discovery™ software version 14.1 (SAS Institute, Cary, NC, USA) was the soft software for the data analysis.
RESULTS
Table 1 shows comparison of haematological parameters in the acute stage of treatment and control groups. In the treatment group, the mean PCV and HGB values were significantly higher compared to the control group. In contract to the PCV and HGB increases, MCHC was decreased in the treatment groups compared to controls. Table 2 shows a significant increase in platelet counts in the treatment groups compared to the control in the chronic stage. Tables 3 and 4 show correlational relationships between some of the studied parameters for the acute and chronic categories. Some of the parameters showed either negative, positive or null relations. Figures 1-12 shows mean variations of studied haematological parameters of acute and chronic categories. The mean concentrations of the parameters investigated exhibited various trends such as a decrease, an increase or no significant difference depending on the duration of exposures to the various concentrations’ of tramadol administered.
| Table 1. Comparisons of Haematological Parameters in the Acute Stage of the Treatment and the Control Groups |
| Parameter |
Experimental Group(N=12)+ |
Test Statistics |
| Treatment (n=6) |
Control (n=6) |
| Mean±SEM |
Mean±SEM |
t- Ratio |
Prob. > |t| |
| PCV (%) |
51.00±2.96a |
37.83±1.47 b |
3.99 |
0.0026** |
| HB (g/dl) |
14.70±0.46 a |
11.55±0.41b |
5.10 |
0.0005*** |
| RBC (x 1012/L) |
24.69±15.87 |
6.81±0.25 |
1.13 |
0.2863ns |
| WBC (x 1012/L) |
13.63±1.15 |
10.35±1.63 |
1.65 |
0.1302 ns |
| MCV (fl) |
60.40±0.26 |
55.83±0.70 |
6.15 |
0.0001 |
| MCH (Pg) |
16.37±0.48 |
16.98±0.33 |
-1.06 |
0.3134 ns |
| MCHC (g/dl) |
28.30±0.52a |
30.43±0.61b |
-2.67 |
0.0237* |
| Platelets (x 109/L) |
756.33±119.94 |
750.67±66.60 |
0.04 |
0.9679 ns |
| Neutrophils (%) |
36.17±3.69 |
41.33±4.06 |
-0.94 |
0.3688 ns |
| Lymphocytes (%) |
53.00±3.08 |
48.67±3.56 |
0.92 |
0.3785 ns |
| Mono (%) |
7.17±1.08 |
7.33±0.76 |
-0.13 |
0.9019 ns |
SEM: Standard error of mean; +Experimental Group:Treatment group–Rats received a lethal dose of Tramadol (Acute Stage), Control group–Rats without Tramadol.
Within parameters, means±SEM with different superscripts are significantly different at p<0.05.
Significance Level: *=p<0.05; **=p<0.01; ***=p<0.0001; ns=Not Significant (p>0.05). |
| Table 2. Comparisons of Haematological Parameters in the Experimental Treatment during the Chronic Stage |
| Parameter |
Experimental Group (N=24) |
Test Statistics |
| 0 mg/kg (Control) |
50 mg/kg |
100 mg/kg |
200 mg/kg |
| Mean±SEM |
Mean±SEM |
Mean±SEM |
Mean±SEM |
F- Ratio |
Prob. > F |
| PCV (%) |
36.33±1.74 |
36.00±1.15 |
34.83±2.12 |
33.17±1.19 |
0.795 |
0.5112ns |
| HB (g/dl) |
11.05±0.37 |
11.05±0.42 |
10.68±0.80 |
1068±0.40 |
0.161 |
0.9215 ns |
| RBC (x 1012/L) |
6.37±0.35 |
6.35±0.24 |
6.23±0.46 |
5.98±0.22 |
0.280 |
0.8389 ns |
| WBC (x 1012/L) |
7.48±1.44 |
6.82±2.12 |
12.92±2.47 |
10.22±0.65 |
2.383 |
0.0998 ns |
| MCV (fl) |
57.2±0.73 |
56.28±1.09 |
56.47±1.10 |
57.28±0.77 |
0.291 |
0.8311 ns |
| MCH (Pg) |
17.48±0.53 |
17.72±0.20 |
17.20±0.34 |
18.02±0.19 |
1.006 |
0.4107 ns |
| MCHC (g/dl) |
30.50±0.66 |
31.30±0.56 |
30.47±0.72 |
31.10±0.35 |
0.514 |
0.6773 ns |
| Platelets (x 109/L) |
549.17±57.66c |
662.50±72.54bc |
828.50±61.77ab |
927.17±28.84a |
8.592 |
0.0007*** |
| Neutrophils (%) |
15.00±1.53 |
15.33±1.15 |
15.83±0.98 |
19.50±2.72 |
1.449 |
0.2585 ns |
| Lymphocytes (%) |
73.67±2.39 |
74.50±2.08 |
74.33±1.41 |
69.33±2.80 |
1.203 |
0.3342 ns |
| Mono (%) |
8.67±0.88 |
6.50±1.09 |
6.00±0.93 |
6.83±0.70 |
1.628 |
0.2146 ns |
| Eosin (%) |
4.33±0.42 |
3.67±0.33 |
3.67±0.21 |
4.00±0.37 |
0.873 |
0.4715 ns |
SEM: Standard error of mean;
+ Within parameters, means±SEM with different superscripts are significantly different at p<0.05.
Number per treatment, n=6;
Significance Level: *=p<0.05; ***=p<0.0001; ns=Not Significant (p>0.05). |
| Table 3. Pairwise Correlations of Haematological Parameters following Lethal Dose of Tramadol (Acute Stage) in Rats |
| Parameter |
By Parameter |
Acute Stage |
| Treatment |
Control |
| Correlation |
p value |
Correlation |
p value |
| HB (g/dl) |
PCV (%) |
0.759 |
0.0798 |
0.844 |
0.0346* |
| RBC (x 10^12/L) |
PCV (%) |
0.429 |
0.3960 |
0.948 |
0.0040** |
| RBC (x 10^12/L) |
HB (g/dl) |
0.072 |
0.8924 |
0.856 |
0.0296* |
| PLATELET (x 10^9/L) |
WBC (x 10^12/L) |
0.817 |
0.0472* |
-0.145 |
0.7840 |
| NEUT (%) |
WBC (x 10^12/L) |
-0.833 |
0.0397* |
-0.199 |
0.7058 |
| NEUT (%) |
PLATELET (x 10^9/L) |
-0.834 |
0.0392* |
0.210 |
0.6891 |
| LYMP (%) |
WBC (x 10^12/L) |
0.926 |
0.0080** |
0.126 |
0.8125 |
| LYMP (%) |
PLATELET (x 10^9/L) |
0.851 |
0.0318* |
-0.106 |
0.8415 |
| LYMP (%) |
NEUT (%) |
-0.957 |
0.0028** |
-0.986 |
0.0003*** |
| Significance Level: *=p<0.05; **=p<0.01; ***=p<0.001; ****=p<0.0001 |
| Table 4. Pairwise Correlations of Haemato-Immunological Parameters following Experimental Treatment with Tramadol in Rats (Chronic Stage)e |
| Parameter |
By Parameter |
Experimental Group (N=24) |
| 0 mg/kg (Control) |
50 mg/kg |
100 mg/kg |
200 mg/kg |
| Correlation |
p value |
Correlation |
p value |
Correlation |
p value |
Correlation |
p value |
| HB (g/dl) |
PCV (%) |
0.911 |
0.0116** |
0.792 |
0.0602 |
0.958 |
0.0026** |
0.931 |
0.0070** |
| RBC (x 10^12/L) |
PCV (%) |
0.997 |
<.0001**** |
0.831 |
0.0405* |
0.977 |
0.0008*** |
0.791 |
0.0609 |
| RBC (x 10^12/L) |
HB (g/dl) |
0.921 |
0.0091** |
0.896 |
0.0157** |
0.972 |
0.0012*** |
0.803 |
0.0541 |
| WBC (x 10^12/L) |
HB (g/dl) |
0.541 |
0.2677 |
-0.267 |
0.6085 |
-0.841 |
0.0359* |
0.420 |
0.4070 |
| MCV (fl) |
PCV (%) |
-0.815 |
0.0484* |
-0.190 |
0.7185 |
-0.614 |
0.1951 |
0.624 |
0.1853 |
| MCV (fl) |
RBC (x 10^12/L) |
-0.847 |
0.0334* |
-0.690 |
0.1296 |
-0.763 |
0.0774 |
0.243 |
0.6422 |
| MCH (pg) |
PCV (%) |
-0.885 |
0.0191* |
0.551 |
0.2570 |
0.119 |
0.8218 |
0.774 |
0.0706 |
| MCH (pg) |
HB (g/dl) |
-0.620 |
0.1896 |
0.192 |
0.7162 |
0.292 |
0.5740 |
0.903 |
0.0137** |
| MCH (pg) |
RBC (x 10^12/L) |
-0.876 |
0.0221* |
0.341 |
0.5086 |
0.063 |
0.9057 |
0.719 |
0.1074 |
| MCHC (g/dl) |
WBC (x 10^12/L) |
-0.704 |
0.1186 |
0.231 |
0.6600 |
-0.949 |
0.0038** |
-0.728 |
0.1006 |
| MCHC (g/dl) |
MCV (fl) |
0.549 |
0.2595 |
-0.916 |
0.0104** |
-0.613 |
0.1956 |
-0.036 |
0.9458 |
| MCHC (g/dl) |
MCH (pg) |
0.940 |
0.0054** |
0.298 |
0.5667 |
0.595 |
0.2128 |
0.114 |
0.8291 |
| PLATELET (x 10^9/L) |
MCHC (g/dl) |
0.854 |
0.0305* |
0.683 |
0.1348 |
-0.235 |
0.6542 |
-0.544 |
0.2648 |
| NEUT (%) |
WBC (x 10^12/L) |
-0.276 |
0.5960 |
-0.864 |
0.0266* |
-0.358 |
0.4859 |
-0.200 |
0.7043 |
| LYMP (%) |
WBC (x 10^12/L) |
0.273 |
0.6008 |
0.863 |
0.0268* |
0.363 |
0.47 |
0.337 |
0.5130 |
| LYMP (%) |
NEUT (%) |
-0.922 |
0.0088** |
-0.841 |
0.0360* |
-0.717 |
0.1086 |
-0.977 |
0.0008*** |
| MONO (%) |
WBC (x 10^12/L) |
-0.817 |
0.0471* |
-0.775 |
0.0703 |
-0.107 |
0.8395 |
-0.789 |
0.0620 |
| MONO (%) |
PLATELET (x 10^9/L) |
0.803 |
0.0546 |
-0.347 |
0.4998 |
0.776 |
0.0695 |
-0.840 |
0.0365* |
| MONO (%) |
LYMP (%) |
-0.074 |
0.8895 |
-0.922 |
0.0089** |
-0.611 |
0.1975 |
-0.553 |
0.2552 |
| EOSIN (%) |
HB (g/dl) |
-0.213 |
0.6854 |
-0.891 |
0.0172* |
-0.243 |
0.6426 |
0.568 |
0.2399 |
| EOSIN (%) |
MCH (pg) |
0.139 |
0.7935 |
-0.184 |
0.7268 |
-0.277 |
0.5952 |
0.847 |
0.0332* |
| EOSIN (%) |
PLATELET (x 10^9/L) |
0.060 |
0.9103 |
-0.878 |
0.0214* |
0.143 |
0.7865 |
0.041 |
0.9383 |
| Significance Level: *=p<0.05; **=p<0.01; ***=p<0.001; ****=p<0.0001 |
|
|
|
|
|
|
|
|
|
|
|
|
Figure 1. Box Plot of PCV in the Control and Treatment Groups During Acute and Chronic Stages
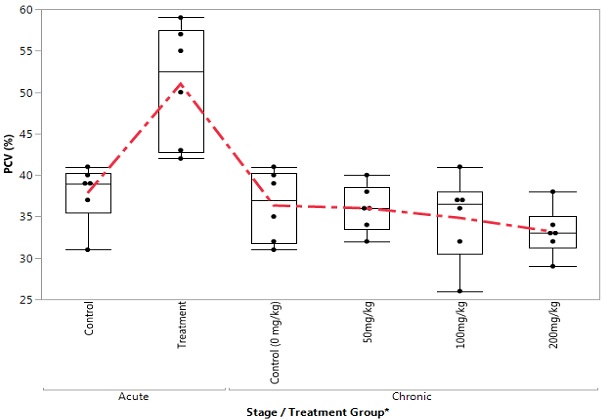
Figure 2. Box Plot of HB in the Control and Treatment Groups during Acute and Chronic Stages
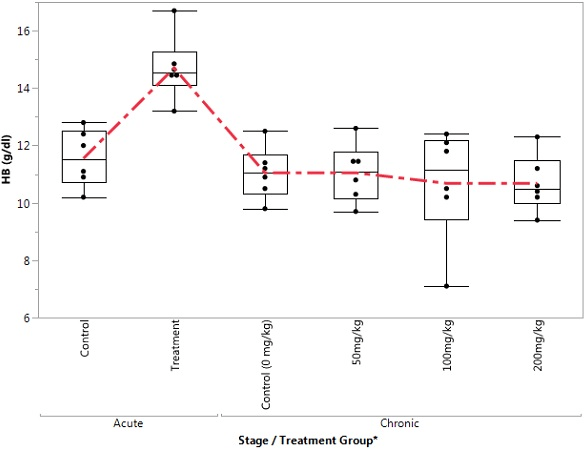
Figure 3. Box Plot of RBC in the Control and Treatment Groups During Acute and Chronic Stages
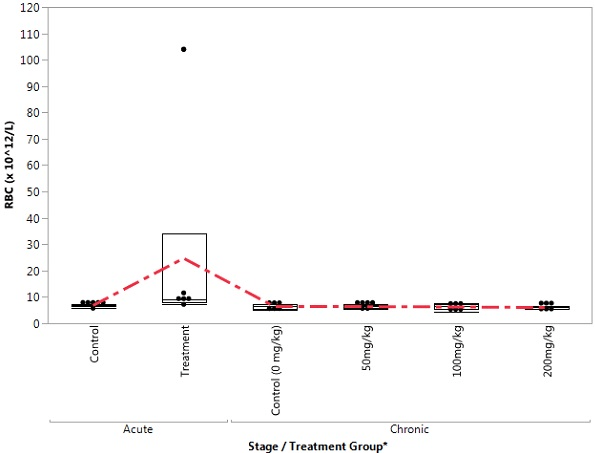
Figure 4. Box Plot of WBC in the Control and Treatment Groups During Acute and Chronic Stages
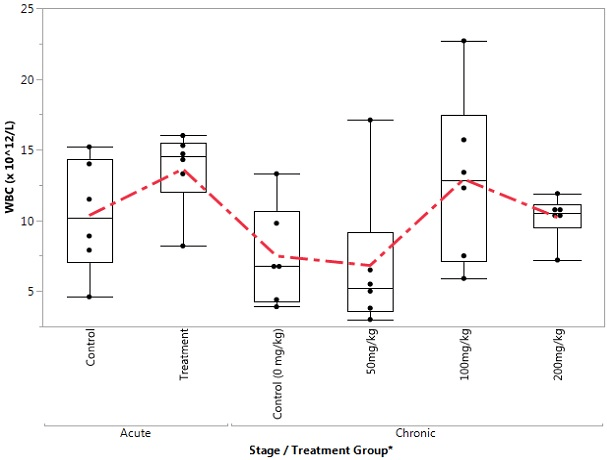
Figure 5. Box Plot of MCV in the Control and Treatment Groups during Acute and Chronic Stages
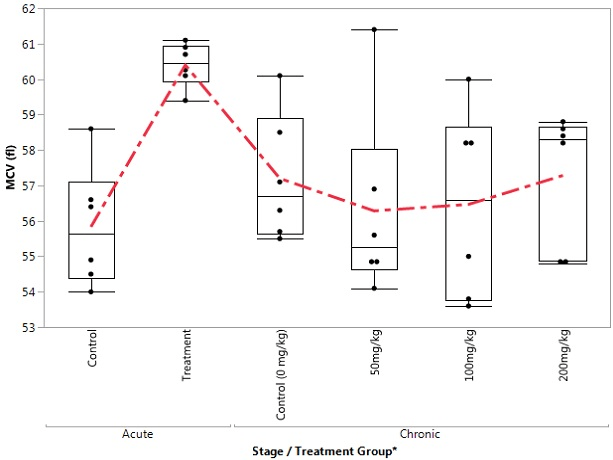
Figure 6. Box Plot of MCH in the Control and Treatment Groups during Acute and Chronic Stages
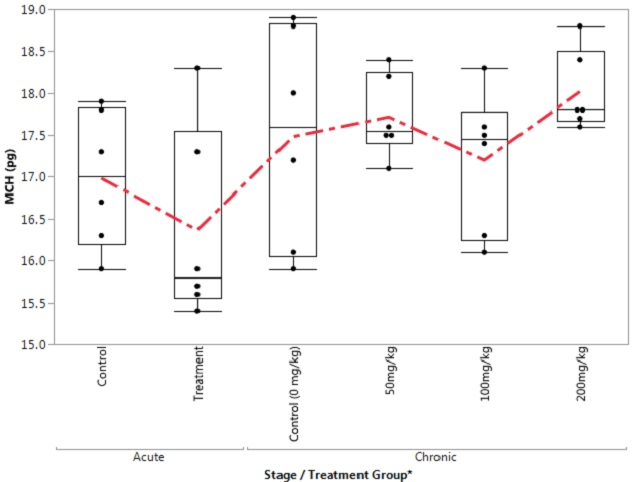
Figure 7. Box Plot of MCHC in the Control and Treatment Groups during Acute and Chronic Stages
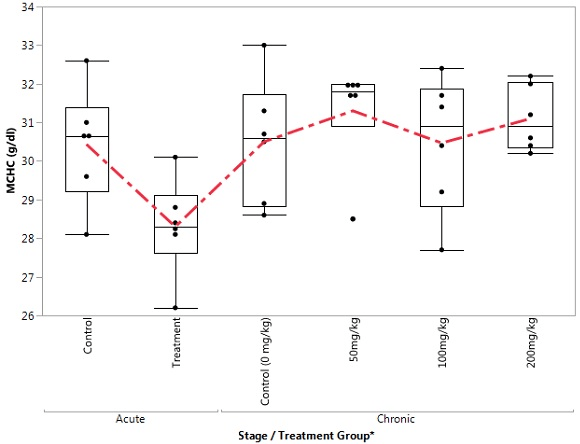
Figure 8. Box Plot of Plateletin the Control and Treatment Groups During Acute and Chronic Stages
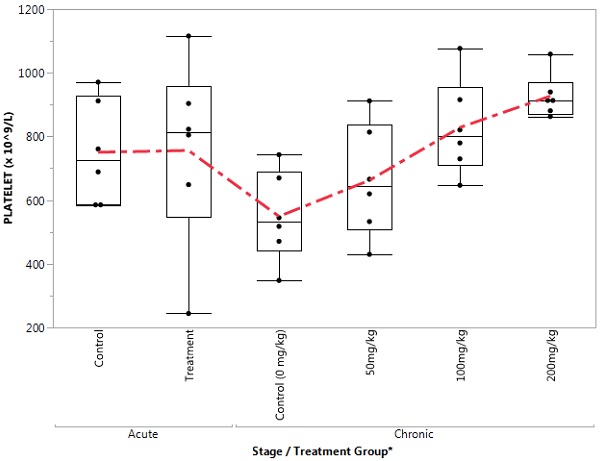
Figure 9. Box Plot of Neutrophil in the Control and Treatment Groups During Acute and Chronic Stages
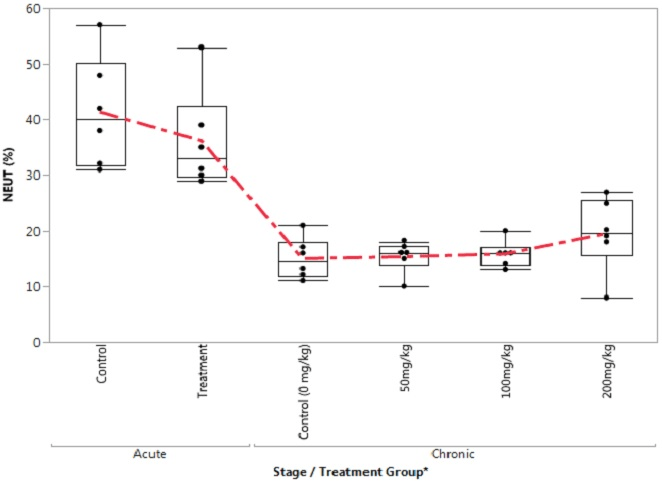
Figure 10. Box Plot of Lymphocytes in the Control and Treatment Groups During Acute and Chronic Stage
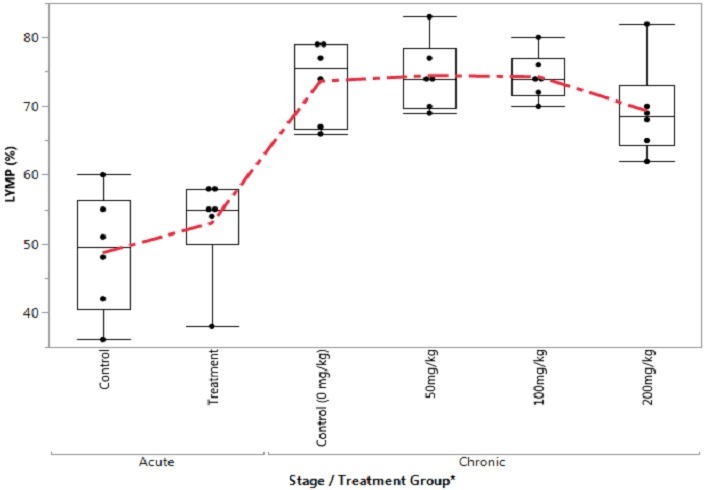
Figure 11. Box Plot of Monophil in the Control and Treatment Groups During Acute and Chronic Stages
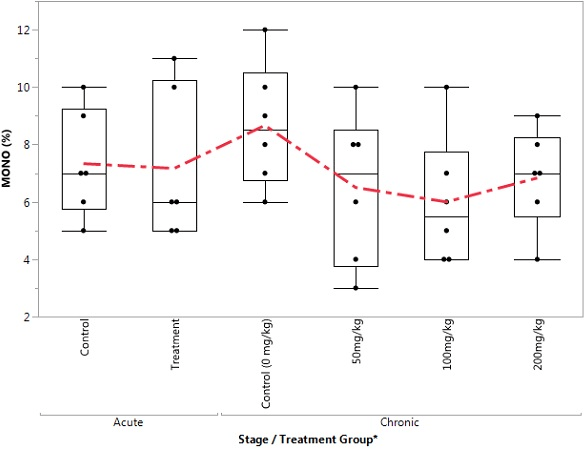
Figure 12. Box Plot of Eosinophil in the Control and Treatment Groups During Acute and Chronic Stages
DISCUSSION
Tramadol addiction and abuse is now a major public health menace in Nigeria. The rampancy of tramadol abuse and the consequential leap in crime in Nigeria led to the Federal Government banning the manufacture, sale and use of tramadol in 2018. The design of the current study investigated the short- and long-term implications of tramadol abuse on haematological parameters and red cell indices using an animal model.
The acute study showed a significant increase in haemoglobin (Hb) concentration and PCV level, whereas the MCHC decreased in the experimental group when compared with the control. Increased concentration of Hb and PCV could be due to changes in trace element concentration. Alterations in trace elements, such as iron, could disrupt the blood cell physiology of the erythrocyte. This bias is also advanced by Guzel et al.20 Haghpanah et al21 that revealed a significant increase in Hb and PCV among persons withdrawing from opium addiction in Iran. However, the pattern of Hb and PCV levels in this study disagreed with that of Goldstein et al22 and Mohammed et al.23
A chronic toxicity study revealed a rise in concentration of platelets as the grades of intoxication increases. This observation could be due to stimulation of platelets progenitors such as pluripotent stem cell, mixed myeloid progenitor cell or/and megakaryocyte progenitor leading to the synthesis of more platelets. There are only two animal studies that have assessed the effect of tramadol on coagulation. A trial in rabbits demonstrated that tramadol and droperidol enhance the coagulation of plasma proteins and suppress the thrombocyte deaggregation process.24 However, in another animal model, it was demonstrated that tramadol was not associated with significant changes in primary haemostasis.25
There are several mechanisms advanced for the increase in platelets count resulting from the use of tramadol. First, tramadol displays anti-inflammatory effects1,26; this mechanism is unclear. It may be because of cyclooxygenase inhibition, which also exerts an anticoagulant effect. Secondly, tramadol and morphine exert similar action on α-2 adrenoreceptors.27,28 Hsiao et al27 mentioned that morphine potentiates agonist-induced platelet aggregation by activating α-2 adrenoreceptors and the mobilization of intracellular calcium. Lastly, another mode of tramadol action is a local anesthetic effect.1 Local anesthetics may induce changes in the membrane of platelets, inhibiting alpha-granule release, thromboxane A2 signaling and aggregation by blocking membrane ion channels.29 Tramadol may also exert effects on membrane ion channels, similar to local anesthetics.29 However, all of these mechanisms are assumptive and need further investigation. Our study does not establish a clear mechanism of action for Tramadol in increasing platelet count, but we observed that Tramadol has a stimulatory effect mechanism on platelet production.
The observed correlations between Hb and PCV, RBC and PCV, RBC and Hb could be hinged on the fact that the syntheses of all these parameters are derived from haemoglobin. Haemoglobin is a protein present mainly in red blood cells of almost all vertebrates. Haematocrit, on the other hand, is a measurement related to total blood count. Both of these are used to diagnose anaemia and hence mistaken to be the same thing frequently. Haemoglobincount is a part of haematocrit and it constitutes the basis of red blood cells. Red blood cell and haemoglobin are inseparable. An effect on the haemoglobin will advertently affect the PCV and RBC. Hence, a non-distortion in the correlation could discriminate acute tramadol intoxication from other similar intoxications that share similar diagnosis and prognosis. The correlation findings of Hb and PCV are also in consonance with the values revealed by Iwalokun et al,30 Omoti31 and Sagir et al.32
This study has further elucidated the acute and chronic effect tramadol intoxication on some haematological parameters which are important in diseases and toxicities diagnosis. The pattern presented of platelets count needs a further research on its therapeutic importance. Furthermore, a study on the effect of tramadol intoxication on coagulation profile and inflammatory markers is crucial in buttressing the scientific basis of some of the alterations noted in this study.
CONCLUSION
The study has shown that tramadol intoxication has alterative propensity on some blood cells and red cell indices, which could be beneficial or deleterious depending on the concentration administered and the duration of exposure. Administration of intolerable concentration of tramadol led to the death of the experimental animals with a consequential significant increase in Hb and PCV levels, whereas a decrease in mean cell haemoglobin concentration (MCHC) was observed. Chronic intoxication of tramadol revealed an increase in platelet counts. “Furthermore, the increased platelet count observed could be therapeutically important to patients having thrombocytopenia dysfunction, coagulation abnormalities, or nearing major surgery. The need for a further study on the mechanism of chronic tramadol intoxication leading to an increase in platelet counts is advocated. Similarly, studies involving humans or a human cell line model would expand our understanding of Tramadol effects on humans.”
ACKNOWLEDGEMENTS
Special appreciation to my project students, staff of the Pharmacology Department of the Niger Delta University, Wilberforce Island, Bayelsa State, Nigeria and that of the Enis Biomedicals and Forensics LTD, Adibon, Igbogene Epie, Bayelsa State, Nigeria.
DECLARATIONS
Ethics Approval
Approval for the study was approved by the Ethics Committee of the Bayelsa State Ministry of Health, Bayelsa State, Nigeria. Standards guiding animal care were adequately followed.
Adherence to National and International Regulations
Not applicable
Consent for Publication
Not applicable
Availability of Data and Materials
Data are available for any interested researcher as far as the source will be acknowledge and credit accorded.
Funding
No funding was granted from any source.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.

















