INTRODUCTION
Now-a-days the use of pesticides in agriculture has been increasing continuously. The harmful effects of many pesticides, such as organophosphates, organochlorine and carbamates, have led to use of pyrethroids as alternatives. Pyrethroids analogs of naturally occurring pyrethrins is widely used in agriculture in many countries because pyrethroids are highly effective, low toxic to non-target organisms and have easy biodegradability.1 Lambda-cyhalothrin (LCT) is a potent, synthetic, type II pyrethroid pesticide and is worldwide used to control a different variety of insect pests in agricultural and domestic fields and public health sectors.2,3,4 It is reported that lambda-cyhalothrin is moderately toxic5,6 for mammals and highly toxic for fish, bees and aquatic invertebrates at low concentrations.7,8 Through the use of agricultural foodstuff, pesticide residues have the ability to affect directly on human health.9 People those are living in proximity to farms and exposed heavily to the home application of pesticides or eat foods rich in pesticide residues, are highly vulnerable to pesticides intoxication in addition to the workers of pesticides manufacturers, agriculture workers and their families.10 It is well documented that the accidental poisonings and death of humans occurred by the use of pesticides especially in developing countries.11
Many pesticides generate their toxicity through the induction of oxidative stress.12 The free radicals generate oxidative damage to all biomolecules and initiate a chain reaction that leads to damage in physiological systems and accumulating free radicals over a period of time cause degenerative diseases.13 Several research studies reported the induction of oxidative stress by synthetic pyrethroids such as fenvalerate and cypermethrin.14,15,16
Taurine possesses antioxidant and membrane-stabilizing properties. Several studies reported that taurine exhibited protective activity against renal toxicity by its antioxidative role.17 On the other study, it was reported that taurine supplementation became resistant to kidney damage and also proteinuria caused by either streptozotocin-induced type 1 diabetes or aminonucleoside-induced glomerulopathy.18 In a related study, chronic taurine treatment prevented aging-related up regulation of transforming growth factor beta (TGF-β1), collagen types I and IV and fibronectin messenger ribonucleic acid (mRNAs), proteins involved in the development of renal fibrosis in aging rat.19 Renal function, especially the oxidative status of the renal system may be altered due to the exposure of pyrethroids. The present study was conducted to evaluate the toxic effect of orally administered lambda-cyhalothrin on renal lipid peroxidation and antioxidant status in male Wistar rat and to find out the ameliorative potential of taurine in this toxic condition.
MATERIALS AND METHODS
Chemicals and Reagents
Lambda-cyhalothrin 5% emulsifiable concentrate (EC) was procured from RPC Agro Industries, Kolkata. Taurine, 1, 2dichloro-4-nitrobenzene (CDNB) were purchased from Sigma-Aldrich. Thiobarbituric acid, 5, 5’ Dithiobis-2-nitrobenzoic acid (DTNB), ethylene di tetraacetic acid (EDTA), and hydrogen peroxide were all purchased from Sigma Chemical, USA. All other chemicals used were of the finest analytical grade.
Animal Care and Treatment
In this study, 36 mature male albino rats (Wistar) weighing 130±15 g were acclimatized for 1 week before the start of the treatments at a suitable temperature of 25 °C±2 °C with 12 hours light-dark cycle. Animals were provided with accessible dry food pellets and water sufficiently. Rats were randomly divided into six groups, and each group contains six animals. Institutional Animal Ethical Committee approved the experimental protocol. The experimental six groups were designed as:
Group-I: DW-Control (Distilled Water, 2 ml/kg body weight)
Group-II: TAU-Control (TAU, 50 mg/kg body wt.)
Group-III: LCT-Low (LCT, 10.83 mg/kg body wt.)
Group-IV: TAU+LCT-Low (TAU, 50 mg/kg body wt.+LCT, 10.83 mg/kg body wt.)
Group –V: LCT-High (LCT, 15.17 mg/kg body wt.)
Group-VI: TAU+LCT-High (TAU, 50 mg/kg body wt.+LCT, 15.17 mg/kg body wt.)
Two respective doses 10.83(1/7th LD50 dose) and 15.17(1/5th LD50 dose) mg/kg body wt. of LCT were applied.20 After one hour of the treatment of taurine (50 mg/kg body wt.), lambda-cyhalothrin was administered at two dose levels (10.83 mg/kg body wt, and 15.17 mg/kg body wt.) for consecutive 14 days. Animal’s weight was taken daily and the dose was adjusted accordingly to weight.
Sample Collection
The total body weight of each animal was recorded at the end of the experimental period. All rats were sacrificed by rapid decapitation after 24 hours of the last dose. Then weights of the kidney tissues were recorded and stored properly for the determination of oxidative stress biomarkers.
Estimation of Renal Index
Renal index was measured by using the following formula
Kidneys weight (g)
Renal index = —————————— x 100
Body weight (g)
Estimation of Serum and Tissue Protein
Different dilutions of BSA solutions are prepared by mixing stock BSA solution (1 mg/ ml) and water. From these different dilutions, protein reagents (98:1:1) consisting of sodium carbonate (Na2CO3) in 0.1 N sodium hydroxide (NaOH), sodium potassium tartrate in distilled water, copper sulphate (Cu2SO4) in distilled water were added to different test tubes and 10 µl of serum or tissue homogenate and 500 µl of normal saline (0.9 g%) were also added. The solutions were mixed well. Then 500 µl of Folin-Ciocalteau solution was added to each tube and incubated at 37 °C for 30 min. The standards were prepared similarly. The optical density was measured at 660 nm.21 The absorbance was plotted against protein concentration to get a standard calibration curve.
Estimation of Oxidative Stress Parameters
Malondialdehyde (MDA): MDA of kidney tissue homogenate was assayed by using the method of Ohkawa et al.22 One ml of homogenate (20 mg/ml phosphate buffer) was mixed with 0.2 ml of 8.1% sodium dodecyl sulphate, 1.5 ml of acetate buffer (20% pH 3.5), and 1.5 ml of aqueous solution of thiobarbituric acid (0.8%). Red pigment was produced after heating of that mixture at 95 °C for 60 min. Then it was extracted with 5 ml of n-butanol-pyridine mixture (15: 1) and centrifuged at 5000 rpm for 10 min at room temperature. The absorbance of the supernatant was measured at 535 nm.
Reduced glutathione (GSH): The reduced glutathione in kidney tissue homogenate was measured according to the method of Griffith.23 The assay mixture contained 200 μl of kidney tissue homogenate and 100 μl of sulfosalicylic acid (4 gm %). The mixture was centrifuged for 10 min at 3000 rpm. Then 1.8 ml of DTNB (4 mg %) was added with the supernatant and was shaken well. Reading was taken at 412-420 nm.
Oxidized glutathione (GSSG): Oxidized glutathione of kidney tissue homogenate was measured by using the method of Griffith.24 At first, 100 μl of kidney tissue homogenate was mixed with 2 μl of 2-vinyl pyridine and was incubated for 1h at 37 °C. Then 250 μl of sulfosalicylic acid (4 gm %) was added with it and was kept in room temperature for 30 min. It is centrifuged at 2000 rpm for 10 min. Then 200 μl of the supernatant was added with 2 ml of DTNB (4 mg %) and the reading was taken at 412 nm within 1min.
Catalase (CAT): CAT content was measured according to the method of Aebi.25 The reaction mixture consisted of 0.5 ml of H2O2, 2.5 ml of double distilled water and 40 μl of kidney tissue homogenate prepared in 0.05 M trisHCl and was taken in a cuvette. After mixing, six readings were noted at 240 nm in 30 sec interval.
Glutathione peroxidase (GPx): Glutathione peroxidase was assayed according to Rotruck et al.26 At first, homogenates (0.2 ml) were mixed with 0.1 ml of 2.5 mM hydrogen peroxide (H2O2), 0.2 ml of 0.4 M sodium phosphate buffer, 0.1 ml of 10 mM sodium azide and 0.2 ml of 4 mM reduced glutathione and was incubated for 5 min at 37 °C. After that 0.4 ml of 10% trichloroacetic acid (TCA) was added to that mixture to stop the reaction and centrifuged at 3200 rpm for 20 min. Then 3 ml of disodium hydrogen phosphate (Na2HPO4) and 1 ml of 5, 5′-dithiobisnitrobenzoic acid (DTNB) were added to 0.5 ml of supernatant.
Statistical Analysis
All data were analyzed by One-Way analysis of variance (ANOVA) followed by two-tail t-test using the Origin 6.0 Scientific Data Analysis. The results were expressed as the Mean±Standard Error of Mean (SEM). The difference between group means was considered significant when p<0.05.
RESULTS
Effects on Renal Index
Results are expressed as Mean±SEM (N=6). The analysis is done by ANOVA followed by multiple comparison two-tail t-tests. Superscript a: Group-I versus all other groups; Superscript b: Group-III versus Group-IV; Superscript c: Group-V versus Group-VI. Asterisks represent the different level of significance (** indicates p<0.01, *** indicates p<0.001).
The renal index of LCT exposed rats was decreased significantly (p<0.001) in a dose-dependent manner compared to a control group (Table 1). Taurine increased the renal index of LCT induced rats significantly.
| Table 1. Effect of Taurine on Renal Index in Lambda-Cyhalothrin Exposed Male Albino Rat |
| Experimental Groups |
Renal Index |
| Group-I: DW-Control (Distilled Water, 2 ml/kg body wt.) |
0.788±0.029 |
| Group-II: TAU Control (TAU, 50 mg/kg body wt.) |
0.799±0.025 |
| Group-III: LCT-Low(LCT, 10.83 mg/kgbody wt.) |
0.677±0.012a** |
| Group-IV: TAU+LCT-Low(TAU, 50mg/kg body wt.+LCT,10.83 mg/kg body wt.) |
0.758±0.018b** |
| Group-V: LCT-High(LCT, 15.17 mg/kg body wt.) |
0.569±0.023a*** |
| Group-VI: TAU+LCT-High (TAU, 50mg/kg body wt. +LCT, 15.17 mg/kg body wt.) |
0.729±0.035c** |
Effects on Kidney and Serum Protein Content
In the present study, lambda-cyhalothrin caused a reduction in total kidney protein level compared to control rats in a dose-dependent manner. At the same time, LCT increased the serum protein level in LCT-exposed rat. Taurine restored back the respective protein levels towards normal in both of the cases (Figures 1 and 2).
Figure 1. The Effect of Taurine on Kidney Tissue Protein in Lambda-cyhalothrin Exposed Male Albino Rat.
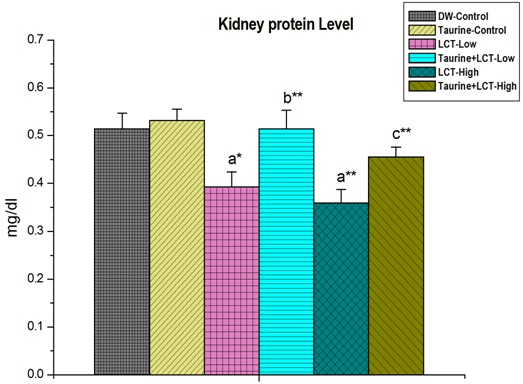
Results are Expressed as Mean±SEM (N=6). Analysis is Done by ANOVA Followed by Multiple Comparison Two-Tail t-tests. Superscript a, Group-I versus all Other Groups; Superscript b Group-III versus Group-IV; Superscript c Group-V versus Group-VI. Asterisks Represent the Different Level of Significance (* indicates p<0.05, **indicates p<0.01)
Figure 2. The Effect of Taurine on Serum Protein in Lambda-cyhalothrin Induced Male Albino Rat.
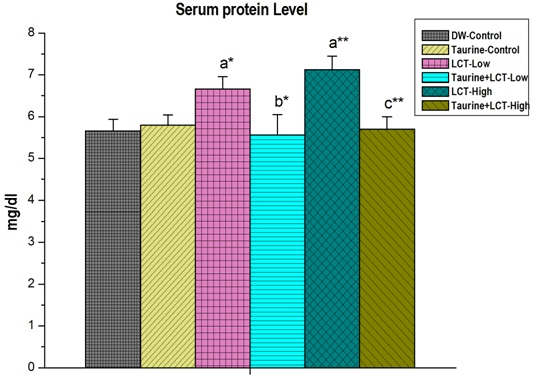
Results are Expressed as Mean±SEM (N=6). Analysis is Done by ANOVA Followed by Multiple Comparison Two-Tail t-tests. Superscript a, Group-I versus all Other Groups;
Superscript b Group-III versus Group-IV; Superscript c Group-V versus Group-VI. Asterisks Represent the Different Level of Significance (* indicates p<0.05, **indicates p<0.01)
Effects on Enzymatic Parameters for Lipid Peroxidation
The effect of taurine on kidney malondialdehyde (MDA) level of lambda-cyhalothrin exposed male albino rat is shown in Figure 3. In LCT treated group, MDA content increased significantly (p<0.001) compared to the control group in a dose-dependent manner where treatment of taurine decreased the LCT toxicity and restored the normal level of the MDA to a great extent.
Figure 3. The Effect of Taurine on Kidney Malon-di-Aldehyde (MDA) Level in Lambda-cyhalothrin Exposed Male Albno Rat.
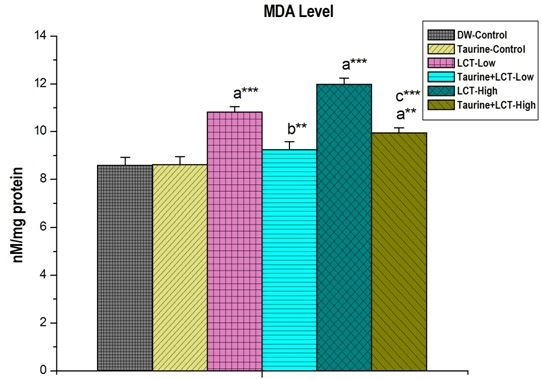
Results are Expressed as Mean±SEM (N=6). SuperScript a, Group-I versus all Other Groups; Superscript b Group-III versus Group-IV;
Superscript c Group-V versus Group-VI. Asterisks Represent the Different Level of Significance (**indicates p<0.01,*** indicates p<0.001)
Kidney GSH level was decreased in LCT low and high dose treated animal groups significantly (p<0.001) but pre-treatment of taurine causes significant (p<0.001) elevation in GSH level in LCT intoxicated animals (Figure 4).
Figure 4. The Effect of Taurine on Kidney GSH Level in Lambda-cyhalothrin Exposed Male Albino Rat.
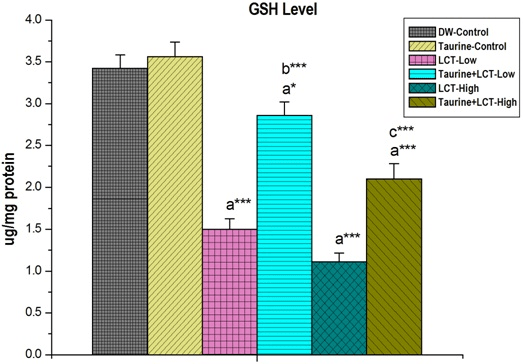
Results are Expressed as Mean±SEM (N=6). Analysis is Done by ANOVA
Followed by Multiple Comparison Two-Tail t-tests. Superscript a, Group-I versus all Other Groups;
Superscript b Group-III versus Group-IV; Superscript c Group-V versus Group-VI.
Asterisks Represent the Different Level of Significance (* indicates p<0.05, *** indicates p<0.001))
Figure 5 shown that the kidney GSSG level in LCT low and high dose treated rats were increased significantly (p<0.05 and p<0.01) compared to control rats. GSSG level was decreased by taurine pre-treatment in LCT exposed rats.
Figure 5. The Effect of Taurine on Kidney GSSH Level in Lambda-cyhalothrin Exposed Male Albino Rat.
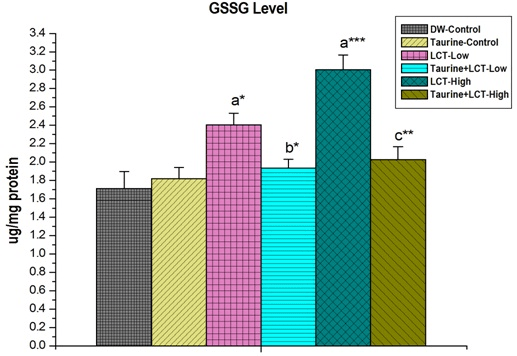
Results are Expressed as Mean±SEM (N=6). Analysis is Done by ANOVA Followed by Multiple Comparison Two-Tail t-tests. Superscript a, Group-I versus all Other Groups;
Superscript b Group-III versus Group-IV; Superscript c Group-V versus Group-VI. Asterisks
Represent the Different Level of Significance (*indicates p<0.05,**indicates p<0.01,***indicates p<0.001)
Effects on Antioxidant Enzymes
As presented in Figure 6, the activities of CAT in the LCT treated low and high dose groups were significantly (p<0.001) decreased compared to the control group. However, the activity of CAT was significantly increased by taurine pre-treatment in low (p<0.01) and (p<0.05) high dose group animals.
Figure 6. The Effect of Taurine on Kidney Catalase (CAT) in Lambda-cyhalothrin Exposed Male Albino Rat.
Results are Expressed as Mean±SEM (N=6). Analysis is Done by ANOVA
Followed by Multiple Comparison Two-Tail t-tests. Superscript a, Group-I versus all Other Groups; Superscript b Group-III versus Group-IV; Superscript c Group-V versus Group-VI.
Asterisks Represent the Different Level of Significance (**indicates p<0.01, *** indicates p<0.001)
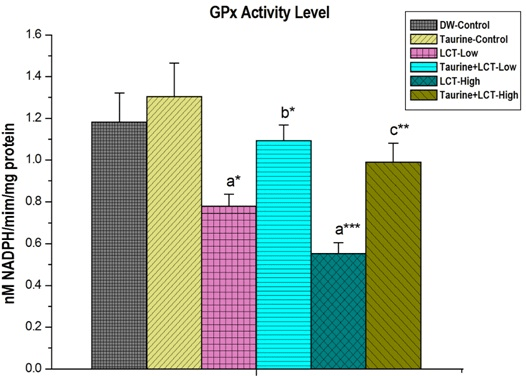
Activities of glutathione peroxidase (GPx) in kidney of LCT treated low and high dose animals were significantly (p<0.05) and (p<0.001) decreased than the control group rats. Taurine treatment significantly increased GPx levels, in low dose (p<0.05) and high dose (p<0.01) animals (Figure 7).
Figure 7. The Effect of Taurine on Kidney Glutathione Peroxidase (GPx) in Lambda-cyhalothrin Exposed Male Albino Rat.
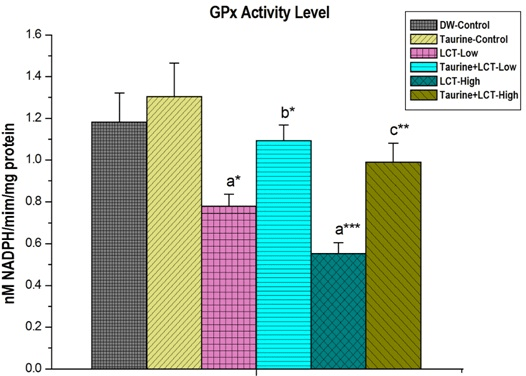
Results are Expressed as Mean±SEM (N=6). Analysis is Done by ANOVA Followed by Multiple Comparison Two-Tail t-tests. Superscript a, Group-I versus all Other Groups; Superscript b Group-III versus Group-IV; Superscript c Group-V versus Group-VI. Asterisks Represent the Different Level of Significance (* indicates p<0.05,**indicates p<0.01)
DISCUSSION
The present study was designed to evaluate the toxic effects of lambda-cyhalothrin on male albino rat kidney and its attenuation by taurine. It has been reported that in toxicological studies, body and organs weights are considered as important criteria for evaluating organ toxicity. The body weight change is considered as a sign of toxicity of any chemical substance.27 In this study, we have evaluated the renal index as the basic marker for the renal toxicity. The renal index of lambda-cyhalothrin intoxicated rats was significantly lower than control rats but attenuation of renal index by taurine was seen and this may be due to its antioxidant activity. Ingestion of chlorpyrifos, diazinon and their mixture resulted in the reduction in relative kidney weight in male rats which is in agreement with our findings.28 In addition; one of the most common reasons for renal tissue damage is oxidative stress which was observed in lambda-cyhalothrin exposed rats.
Proteins, the essential organic macromolecules for cellular structure and function, are expected to react first with pesticide after the entry of pesticide into body cells. Pesticides have the ability to alter very quickly the buffering system of the intracellular environment. Pesticides impair protein metabolism leading perhaps to a disarray of functional and structural status of the cell.29 In the present study, lambda-cyhalothrin had a significant lowering effect on total serum protein levels in dose-dependent manner compared to control rats.
Toxicants can cause a defect in protein synthesis and that may lead to a decrease in tissue protein content. The exposure of toxins to living organisms may alter the hormonal balance that can results a direct or indirect decrease in tissue protein content.30,31 The effect of different pesticides such as endosulfan,32 organchlorins,33 chlorpyrifos,34 phosphorothionate,35 imidacloprid,36 and cypermethrin37 poisoning on protein metabolic profiles of rats has been studied by different researchers. In current investigation exposure of lambda-cyhalothrin to albino rats resulted in a gradual decrease in the protein content of kidney tissue.
Oxygen free radical induced lipid peroxidation, which causes damage to cell membranes and consequently develops tissue injury.38 In this study, lambda-cyhalothrin elevated renal malondialdehyde (MDA) level and reduced renal glutathione (GSH) contents as well as inhibited glutathione-s-transferase activity of the kidney tissue. The decrease in tissue GSH due to the enhancement in lipid peroxidation is considered as an antioxidant defence role of GSH. GSH, glutathione-s-transferase are used in the cell as antioxidant defence mechanism. Reduced glutathione serves as an antioxidant against free radicals and organic peroxide.39
LCT induced toxic manifestations may also be associated with the induction of oxidative stress through the formation of free radicals and alteration in antioxidant systems. It was observed that LCT significantly increased the level of MDA in the kidneys of rats, whereas the activity of antioxidant enzymes (CAT) was decreased.40 Treatment with taurine caused a significant reduction in the toxic effects of this pesticide. The administration of LCT in different periods of postnatal ontogenesis was also reported to enhance oxidative stress by a significant increase in MDA level and suppressed activity of antioxidant enzymes (CAT) in brain tissue.41 In our study, we found that administration of LCT to rats resulted in a marked dose-dependent increase in the lipid peroxidation as indicated by the increase in the level of malondialdehyde (MDA) and that may be due to LCT induced increase in ROS level. GSH, one of the most important biological molecules, play a key role in the detoxification of the reactive toxic metabolites. Decline in GSH levels in the kidney after LCT treatment may be an indication of oxidative stress, whereas GSH is utilized for the detoxification of reactive toxic substances. An increased level of GSSG also reflects the oxidative stress of ovary. Normal cellular functioning depends on a balance between ROS production and antioxidant defence mechanisms present in the cell.
Antioxidant enzymes cause a primary defence that prevents oxidative damage of biological macromolecules. According to the results, the activities of CAT, a glutathione peroxidase in the kidney of LCT treated rats were significantly decreased. These results suggested that LCT has the capability to induce free radicals and oxidative damage as evidenced by alterations in various antioxidant enzymes.42 Reduction of antioxidant enzymes levels may be due to the direct effect on the enzymes against LCT-induced ROS generation. Taurine administration reversed all these abnormalities of above mentioned renal parameters to a good extent. It diminished lipid peroxidation either by scavenging or quenching oxygen-derived free radicals, hydrogen peroxide or hypochlorous acid directly, or by binding free metal ion species like Fe2+ or Cu2+ by its sulfonic acid group. It was also suggested that by decreasing carbonyl group production, enhanced oxidative damage43 was reduced by taurine.17
CONCLUSION
The present findings demonstrated that taurine was able to reverse the pathological parameters of renal damage induced by lambda-cyhalothrin. Pre-treatment of taurine maintained the antioxidant status of kidney due to its free radical scavenging action. Taurine undoubtedly restored the renal function by blocking lambda-cyhalothrin induced renal oxidative stress. So, taurine may be considered useful against lambda-cyhalothrin induced toxicity in renal system.
ACKNOWLEDGEMENTS
The authors would like to thank the authority of Vidyasagar University, Midnapore, India for providing all the facilities to execute this study.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.












