INTRODUCTION
Heavy metals are the main contaminants of soil and water basins. Their high concentration in nature affects the biodiversity and poses a serious threat to human health.1,2 Anthropogenic activity is the general cause underlying the constantly increasing levels of different toxicants including heavy metals. The rapid development of the industry is an important factor attributed to the elevated concentrations of heavy metals in the environment. Direct disposal of industrial wastewater from factories associated with the production of fertilizers, textile, batteries, paper, installations for metal covering and mining, is responsible for causing a significant percent of the pollution on account of heavy metal contamination. In contrast to the organic pollutants, heavy metals are not biodegradable and accumulate in living organisms. Because of their high degree of toxicity, these elements rank among the priority metals that are of great significance to public health. These are all systemic toxicants that are known to induce multiple organ damage, even at lower levels of exposure. Ions of many known heavy metals (zinc, copper, nickel, mercury, cadmium, lead, chromium) are carcinogenic. According to the International Agency for Research on Cancer (IARC), these metals are also classified as either “known” or “probable” human carcinogens based on existing research. Epidemiological and experimental studies demonstrate an association between heavy metal exposure and incidence of cancer in humans.3 Undoubtedly, these pollutants pose a serious threat that needs to be resolved and scientists are actively working to find solutions.4 It is necessary to develop sensitive methods for biomonitoring of heavy metals in the environment in addition to the conventional chemical analysis because essential parameters like bioavailability, cytotoxicity and genotoxicity can be examined only in live cells.
There is a growing interest in the application of algae for the elimination of organic and inorganic contaminants. Biotechnological tools and techniques based on the activity of growing algae cultures could be applied effectively in the process of heavy metal extraction from industrial and wastewater. The ability of microalgae, including species from the genus Scenedesmus, to remove heavy metals from the environment by accumulating these chemicals is currently being investigated.5,6,7
On the other hand, exposition to heavy metals induces synthesis of specific products (pigments, lipids, phytohormones, exopolymers and others) in some algal species. Stimulation of such production depends on many factors: the type of heavy metal and its concentration, strain specificity, culture conditions and growth medium composition.8 A promising new approach is the combination of processes of heavy metal removal and biosynthesis of specific products of interest induced by heavy metals exposition. Many studies are directed to explore the microalgae phenotypic plasticity in response to the exposition to heavy metals. Their general objective is to clarify the potential application of microalgae as bioindicators of this type of pollution.9,10,11,12,13 Species from the genus Scenedesmus (Chlorophyta) are widely spread in freshwater basins and are convenient test-objects for scientific research including the evaluation of the impact of heavy metals.14,15,16 The algal growths, morphology, pigment content, photosynthetic and biochemical activities are affected by heavy metals.
The potential application of algal cultures for the detection of heavy metal pollution is unambiguous. Investigation of specific responses of different algal strains against grading concentrations of heavy metals is needed to enhance our knowledge on algal test-systems and apply it in some biotechnological manufactured products.
In addition to algal cultures, mammalian cell lines present a useful research model for the examination of the mode of action of toxic agents and monitoring their levels. The use of this model organism allows for the visualization of the uptake and distribution of heavy metals, identification of cellular targets and metabolic processes that are affected by the toxicants. Also, it facilitates the examination of the level of response to the toxicant concentration and thus, could serve as a tool for biomonitoring environmental pollution. In cell culture systems, the humoral, neural and other control mechanisms are absent, thus, an examination of the concentration gradient and secondary effects of bioaccumulation are not applicable. Cell lines constitute a homogenous system comprised of specific cell types allowing for the direct investigation of different cellular processes under well-defined and reproducible experimental conditions excluding secondary effects induced in specific locations of the whole organism.17 Mammalian cell lines have been used to analyze the target organelles and cellular molecules for heavy metal toxicity.18 Heavy metals like nickel (Ni), lead (Pb) and cadmium (Cd) have been shown to induce reactive oxygen species production and oxidative stress in cultured mammalian cells.19,20,21 Different types of heavy metal ions have been found to interact with deoxyribonucleic acid (DNA) and nuclear proteins eliciting conformational changes and damage to the key cellular molecules. Overall, these processes affect all the phases of cell cycle but mostly the synthetic phase17 leading to carcinogenesis or apoptosis.18,22 Depending on the studied cell type, specific effects of heavy metals at the cellular level can be elucidated using different cell lines.3
In the present study, the cytotoxic and growth inhibitory effect of cadmium, nickel and lead were analyzed using the two assays—methyl-thiazol-tetrazolium (MTT) test which is based on the ability of metabolically active mitochondria to reduce tetrazolium salts, and Neutral red (NR) assay used to assess cell membrane integrity and cellular viability. These assays were standardized in numerous studies and were used to assess cytotoxicity.23,24,25
The discussed experiments aimed to investigate the impact of increasing concentrations of cadmium, nickel and lead on the green alga Scenedesmus incrassatulus and the human cell lines HeLa, A549, FL, and Caco-2. The choice of these two testsystems allowed for the comparison of the effects of heavy metals on the density and growth of the plant and mammalian cells. The observed results raised interesting conclusions about the influence of Cd, Ni and Pb on the morphology of S. incrassatulus, the affected physiological processes and their targeted structures in both plant and human cells and elucidated their potential application like tools for biomonitoring of heavy metal pollutants as lead, cadmium or nickel.
MATERIALS AND METHODS
Algal Culture and Exposition to Different Concentrations of Heavy Metals
Scenedesmus incrassatulus BOHLIN used in the present study was obtained from the algological collection of the Plovdiv University (Plovdiv Algal Culture Collection, РАСС). The algal strain was stored under the reference number PACC 7069. The algae were grown in ВВМ1-Bold`s Basal Medium26 under optimal growth conditions for the species: 28 °C temperature and light regimen 15:9 hours (light:dark period). A 72-hours intensive cultivation was conducted for the experiment involving exposition to heavy metals. Scenedesmus incrassatulus cultures were grown in BBM1 medium containing different concentrations of Cd2+, Ni2+ and Pb2+. Stock solutions of nitrate salts of each heavy metal (Cd(NO3)2, Ni(NO3)2, Pb(NO3)2) were used. The heavy metal concentrations were selected according to the maximal permissible levels (MPL) established by the Bulgarian law (in accordance with the National regulation for standards of environmental quality for priority substances and some other contaminants), which was based on the European legislation for water quality.27 100%, 75%, 50% and 25% of the MPL for cadmium, lead and nickel were tested in the experiments. Overall, 13 main samples were assayed: control with standard growth medium; growth medium with 0.45 μg/L Cd2+ (corresponding to maximum permissible level /MPL/); growth medium containing 0.3375 μg/L Cd2+ (corresponding to 75% MPL); growth medium with 0.225 μg/L Cd2+ (50% MPL); growth medium containing 0.1 μg/L Cd2+ (25% MPL); growth medium with 20 μg/L Ni2+ (corresponding to MPL); growth medium with 15 μg/L Ni2+ (75% MPL); growth medium containing 10 μg/L Ni2+ (50% MPL); growth medium with 5 μg/L Ni2+ (25% MPL); growth medium with 7.2 μg/L Pb2+ (corresponding to MPL); growth medium containing 5.4 μg/L Pb2+ (75% MPL); growth medium with 3.6 μg/L Pb2+ (50% MPL); growth medium containing 1.4 μg/L Pb2+ (25% MPL). All tested concentrations represent the free heavy metal content corrected from the nitrate formula weight.
All experimental variants were assayed in Ackerman’s vials with 25 ml algal suspension, consisting of 20 ml of growth medium or growth medium plus heavy metal followed by a 5 ml algal inoculum (Density of 200×104 cells/mL).
Measurement of Algal Growth
The growth of algal cultures was determined spectrophotometrically at three time points: 24 h, 48 h and 72 h. Extinction at 663 nm wavelength was measured using the Visible Spectrophotometer M107 (Spectronic Camspec Ltd., Leeds, UK).
Morphological Analysis of Scenedesmus incrassatulus
The morphological analysis was performed on a standard light microscope Magnum-T equipped with high definition digital camera Si-3000 and software (Medline Scientific, Chalgrove, Oxon, UK). The following taxonomical parameters were analyzed at a maximal magnification of 400x: number of cells in the coenobium/single cells, position of the inner cells in the coenobium, morphology of peripheral cells. One hundred coenobia/single cells were examined in order to determine the first taxonomical sign (number of cells). To evaluate the parameters “position of the inner cells” and “morphology of the peripheral cell”, 50 coenobia were analyzed. The morphological variability of S. incrassatulus was documented using the digital camera.
Assessment of Cytotoxicity on Human Cell Lines In Vitro
MTT and NR assays were performed in order to evaluate the toxic effects of Cd2+, Ni2+ and Pb2+. Four different human cell lines were used for performing the experiments, namely: FL (ATCC CCL 62) derived from human amniotic cells; A549 (ATCC CCL 185) isolated from lung carcinoma; HeLa (ATCC CCL-2) established from cervical adenocarcinoma, and Caco-2 (ECACC 86010202) derived from colorectal adenocarcinoma. The cells were incubated in the presence of different concentrations of heavy metals corresponding to 100% MPL, 75% MPL, 50% MPL and 25% MPL for 24, 48 and 72 h at 37 °C, 5% CO2 and high humidity. Stock solutions of nitrate salts of each heavy metal (Cd(NO3)2, Ni(NO3)2, Pb(NO3)2) were added to the cell culture medium (Dulbecco’s modified Eagle’s medium, DMEM) supplemented with 10% fetal calf serum and antibiotics (all from Sigma-Aldrich, Germany) to achieve the appropriate concentration of Cd2+, Ni2+ and Pb2+ in the assayed samples. The control cells were incubated for the same time periods in supplemented DMEM devoid of heavy metals. All samples were assayed in triplicates.
All cell lines were expanded in 75 cm2 culture flasks (TPP, Trasadingen, Switzerland) following which the cells were detached from the culture vessel. The resulting suspensions were adjusted to 1×105 cells/ml concentration and the cells were seeded on 96-well plates (TPP, Trasadingen, Switzerland) using a 200 mL suspension/well. The cells were cultured in standard supplemented DMEM for 24 h at 37 °C, 5% CO2 and high humidity. Then, the culture medium was replaced with complete DMEM containing 100%, 75%, 50%, 25% and 0% MPL of Cd, Ni or Pb. At the end of each test-period (24 h, 48 h, 72 h), MTT (3-(4,5-dimethylthiazol-2-yl)-2,4-diphenyltetrazolium bromide) (Sigma-Aldrich, Germany) solution in a final concentration of 0.5 mg/ml was added to all samples. The cells were incubated for 3 h in a humidified incubator at 37 °C with 5% CO2 content. During this period, live cells with functional mitochondria were able to reduce MTT to an insoluble formazan product. The quantity of accumulated formazan corresponded to the number of viable cells in the sample. Subsequently, the MTT containing medium was removed and 100 mL DMSO were pipetted into each test-well. The cells were incubated for 15 minutes at room temperature on a shaker in order to dissolve the accumulated formazan crystals in the cells. Absorbance was measured at 570 nm wavelength using Synergy-2 reader (BioTek, Winooski, VT, USA). The percent rate of inhibition of cell growth was calculated using the absorbance units from each test sample and the data from the control cells incubated in the absence of heavy metals.
For the Neutral red (NR) assay, FL, HeLa, A549 and Caco-2 cells were expanded, subcultured, seeded on 96-well plates and treated with heavy metals in the same way as described for the MTT assay. The NR stain bound to the lysosomal matrix only in live cells. Therefore, the NR assay was used to evaluate the cell culture viability and growth after treatment with the test-agent. In the described experiments, after 24, 48 and 72 hours treatment with heavy metals, the cells were stained with 0.5 mg/mL NR solution for 3 h at 37 ºC and high humidity. Then, the medium containing unbound stain was aspirated and 100 mL extracting solution (50% ethanol-1% acetic acid) was added to all samples. The culture plates were incubated for 15 min on a shaker and then absorption at 540 nm was measured using Synergy-2 reader (BioTek, Winooski, VT, USA). Similar to the MTT assay, percent inhibition was calculated based on the detected absorbance units.
Statistics
The non-parametric Mann-Whitney U-test was applied to determine statistically significant differences between the assayed samples using StatView software (SAS Institute, USA). The Mann-Whitney U-test compares the median of two groups of data, i.e., control group and treated group. It is used to test the null hypothesis whether two sample groups come from the same population/have the same median and it converts the scores on the continuous variable to ranks, across the two groups. Calculated p values lower than 0.05 were considered statistically significant.
RESULT
Cd2+, Ni2+ and Pb2+ Effects on the Growth and Development of Scenedesmus incrassatulus
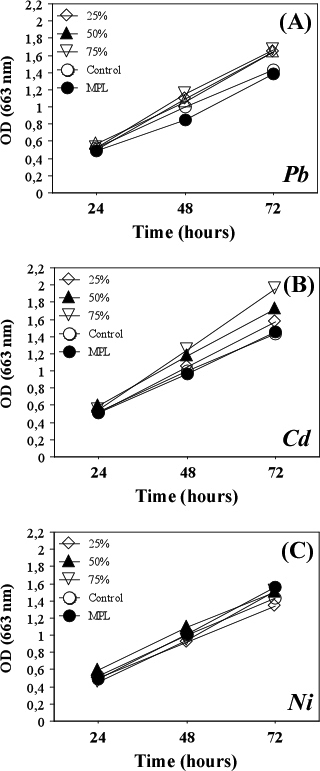
The studied heavy metals exerted different effects on the growth of S. incrassatulus. The presence of lead in the culture medium in concentrations corresponding to MPL inhibited the growth and development of algae (Figure 1A). Low concentrations of cadmium (corresponding to 25%, 50% and 75% of MPL) had a stimulatory effect on S. incrassatulus growth (Figure 1B); however, all tested concentrations of nickel did not affect the density of the algal culture (Figure 1C).
Figure 1: Influence of Lead, Cadmium and Nickel on the Algal Culture Growth.
Cd2+, Ni2+ and Pb2+ Influenced the Morphology of S. incrassatulus
The changes in S. incrassatulus morphology were evaluated by analyzing the taxonomical characteristics “cell number in the coenobium/single cells”, “position of the inner cells in the coenobium” and “shape of the peripheral cell”.
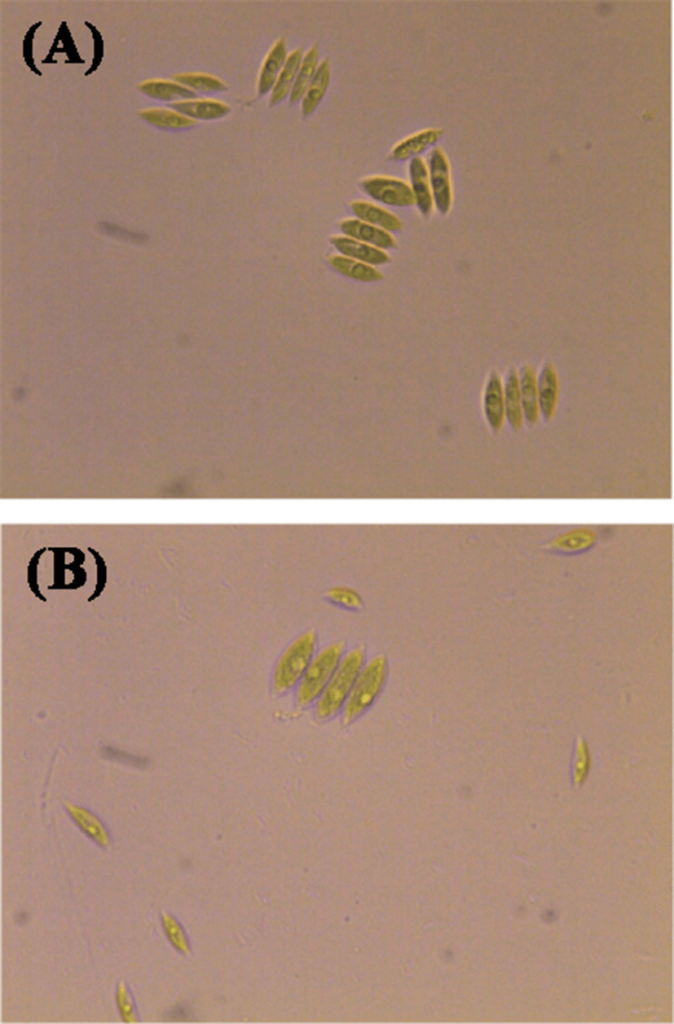
S. incrassatulus reacted to the presence of cadmium and nickel by formation of single cells and reduction of the percent coenobia. These alterations were detected already 24 h after treatment with the heavy metals (Figure 2). The effect was stronger when the algal cultures were treated with 100% MPL concentrations of Cd (Figure 2B) and Ni (Figure 2C). Dissociation to single cells has been shown on Figure 3.
Figure 2: Reaction of S. incrassatulus Concerning the Taxonomical Sign “Cell Number in the Coenobium/Single Cells”.
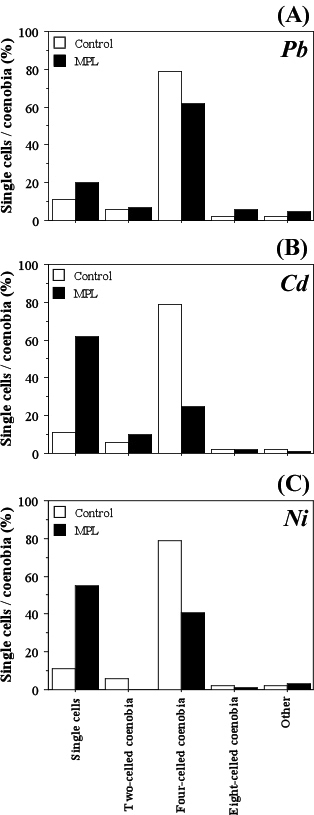
Figure 3: Dissociation of S. incrassatulus to Single Cells after Exposure to Cd. Control Culture (24 h) with
Predominance of Coenobia (A); in Growth Medium with Cd2+ (MPL, 24 h) with Predominance of Single Cells (B).
Cadmium and nickel affected the arrangement of inner cells in coenobia, increasing the percentage of irregularly positioned inner cells compared to the linearly arranged cells. In the presence of nickel, this effect is observed even at 24 hours (Table 1).
| Table 1: Reaction of S. incrassatulus Concerning the Taxonomical Sign “Arrangement of the Inner Cells”. |
| Exposure Time |
Arrangement of Internal Cells |
Control
(%) |
Lead |
Cadmium |
Nickel |
| MPL (%) |
MPL (%) |
MPL (%) |
| 24 h |
Linearly |
58 |
46 |
64 |
26 |
| Alternately |
24 |
10 |
22 |
12 |
| Irregularly |
18 |
44 |
14 |
62 |
| 48 h |
Linearly |
42 |
30 |
8 |
22 |
| Alternately |
28 |
32 |
26 |
28 |
| Irregularly |
30 |
38 |
66 |
50 |
| 72 h |
Linearly |
56 |
26 |
16 |
26 |
| Alternately |
26 |
34 |
28 |
36 |
| Irregularly |
18 |
40 |
56 |
38 |
| MPL: Maximal Permissible Levels; h: Hours. |
The reaction of S. incrassatulus to MPL concentrations of cadmium and nickel 24 and 48 h after treatment concerning the sign “shape of the peripheral cell” led to the predominance of the typical species shape “incrassatulus” (Table 2). On the other hand, 24 h treatment with Pb reduced the type “incrassatulus” and led to an increase in the occurrence of the morphology type “obliquus”. At longer exposition to Pb, percent cells with “other location” were detected while the type “incrassatulus” decreased (Table 2).
| Table 2: Reaction of S. incrassatulus Concerning the Taxonomical Sign “Shape of the Peripheral Cell”. |
| Exposure Time |
Shape of Peripheral Cell |
Control
(%)
|
Lead |
Cadmium |
Nickel |
| MPL (%) |
MPL (%) |
MPL (%) |
| 24 h |
Type”incrassatulus” |
38 |
30 |
56 |
56 |
| Type”obliquus” |
40 |
68 |
30 |
36 |
| Other type |
22 |
2 |
14 |
8 |
| 48 h |
Type”incrassatulus” |
52 |
44 |
60 |
94 |
| Type”obliquus” |
36 |
40 |
26 |
4 |
| Other type |
12 |
16 |
14 |
2 |
| 72 h |
Type”incrassatulus” |
10 |
0 |
6 |
6 |
| Type”obliquus” |
50 |
30 |
74 |
60 |
| Other type |
40 |
70 |
20 |
34 |
| MPL: Maximal Permissible Levels; h: Hours. |
Treatment with Cd2+, Ni2+ and Pb2+ Reduced the Growth and Viability of Human Cell Lines
МТТ and NR assays were performed using different human cell lines. Accumulation of formazan and NR in the cells were measured after three time points (24, 48 and 72 h) of exposition to Cd2+, Ni2+ and Pb2+. The recorded results demonstrated significant cytotoxic effects of heavy metals, which were most prominent at the highest test-concentrations (100% MPL) of Cd2+, Ni2+ and Pb2+ for all cell lines.
А549 cells showed well established dose-dependent response to treatment with different concentrations of heavy metals (Figure 4). The highest percent inhibition of cell growth was determined after treatment with 100% and 75% MPL concentrations already at the shortest exposition period (24 h). The results from the NR assay demonstrated an interesting trend—higher inhibition of cell growth at 24 h test-period compared to the longer exposition times (48 and 72 h). On the other hand, the results from the MTT assay did not show time-dependent level of response. These data indicated a stronger effect of heavy metal exposition on cellular lysosomes, which was partly reduced by developed compensatory mechanisms when the cells were treated for a longer period. Conversely, such tendency was not observed in HeLa cells, which showed the highest percent inhibition and clear dose-dependent response after 72 h treatment with all three heavy metals when measured by NR assay (Figure 5). Caco-2 cells also displayed stronger inhibition after 72 h of exposition measured by MTT assay (Table 3). The NR assay did not show a time-dependent response. Overall, cadmium and lead treatment induced a stronger inhibition of Caco-2 cells compared to treatment with nickel. Interestingly, only the MTT assay results showed time-dependent response with a prominent increase in percentage inhibition after 72 h exposition to heavy metals.
Figure 4: Inhibition of A549 Cells Growth after Treatment with Cadmium, Nickel and Lead.
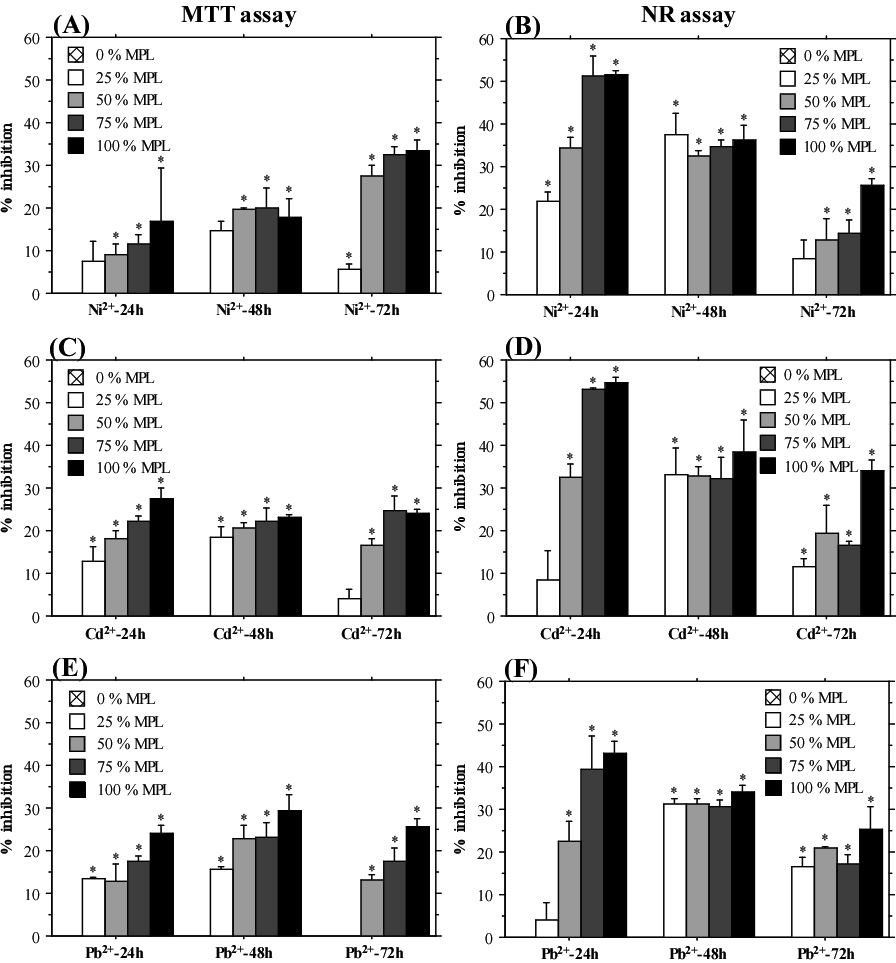
MTT Assay (A), (C), (E); NR Assay (B), (D), (F). The Graphs Display Mean±SE. *p˂0.05.
Figure 5: Influence of Nickel, Cadmium and Lead Exposition on HeLa Cells.
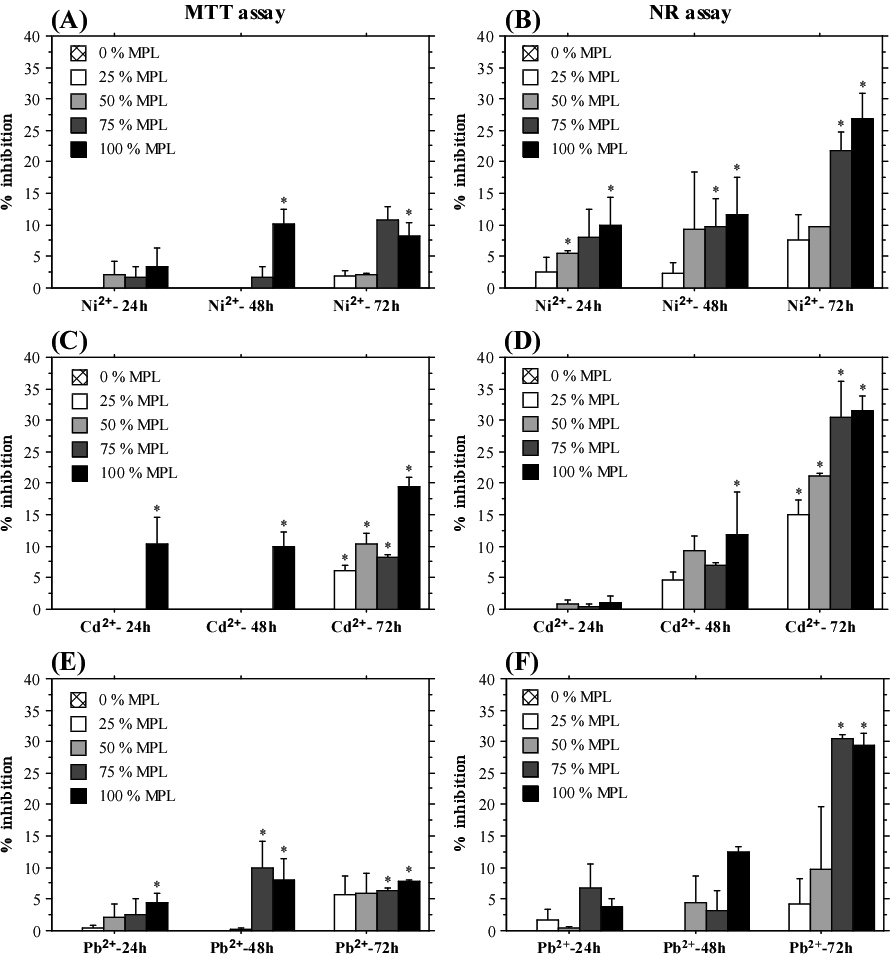
MTT Assay (A), (C), (E); NR Assay (B), (D), (F). The Graphs Show Mean±SE. *p˂0.05.
| Table 3: Inhibition of Caco-2 Cells Growth Induced by Cadmium, Nickel and Lead Treatment. Data has been Displayed as Mean Percent Inhibition. |
| Caco-2 |
Nickel |
Cadmium |
Lead |
| 24 h |
48 h |
72 h |
24 h |
48 h |
72 h |
24 h |
48 h |
72 h |
| MTT assay |
100% MPL |
12.94 |
8.74 |
22.16 |
12.59 |
11.19 |
38.92 |
12.59 |
12.24 |
28.41 |
| 75% MPL |
2.80 |
3.15 |
18.19 |
7.35 |
7.00 |
29.83 |
7.00 |
3.50 |
25.57 |
| 50% MPL |
2.45 |
7.70 |
15.63 |
6.65 |
10.49 |
21.31 |
8.50 |
2.45 |
25.86 |
| 25% MPL |
2.45 |
4.55 |
11.94 |
5.60 |
6.99 |
19.60 |
5.59 |
1.40 |
23.87 |
| NR assay |
100% MPL |
24.60 |
30.67 |
21.09 |
34.20 |
40.10 |
31.96 |
34.60 |
22.48 |
31.96 |
| 75% MPL |
23.20 |
18.56 |
14.13 |
33.20 |
36.41 |
21.74 |
34.00 |
32.59 |
24.57 |
| 50% MPL |
22.60 |
11.92 |
10.87 |
29.20 |
18.63 |
18.27 |
27.60 |
0 |
21.52 |
| 25% MPL |
11.60 |
10.94 |
5.92 |
21.60 |
2.85 |
14.78 |
0 |
5.74 |
13.74 |
| MPL: Maximal Permissible Levels; h: Hours; MTT: methyl-thiazol-tetrazolium; NR: Neutral Red. |
Table 4 presents the data from MTT and NR assays performed with FL cells. The recorded observations demonstrated a higher sensitivity of the NR assay. The highest percent inhibition was induced by exposition to cadmium. MPL concentrations of lead and cadmium induced significant inhibition after 24 h exposition measured by MTT assay, but this effect was not evident at longer test-periods. Therefore, heavy metal toxicity was predominantly directed to cellular lysosomes in the FL cell line similar to A549. Lead and nickel treatment resulted in lower inhibition of cell growth. Like HeLa cells, the strongest percent inhibition of FL and Caco-2 growth was measured after 48 and 72 h exposition to Cd2+. These results demonstrate higher sensitivity of HeLa, FL and Caco-2 cell to cadmium treatment, while A549 cells exhibited similar level of response to all three heavy metals.
| Table 4: Inhibitory Effects on FL Cell Line Determined after Exposition for Different Time-Periods to Nickel, Cadmium and Lead. Data has been Ddisplayed as mean Percent Inhibition. |
| FL |
Nickel |
Cadmium |
Lead |
| 24 h |
48 h |
72 h |
24 h |
48 h |
72 h |
24 h |
48 h |
72 h |
| MTT assay |
100% MPL |
5.29 |
7.28 |
4.97 |
18.81 |
5.55 |
9.15 |
16.18 |
9 |
4.05 |
| 75% MPL |
7.52 |
1.92 |
4.97 |
6.43 |
1.34 |
0.78 |
5.32 |
4.98 |
1.83 |
| 50% MPL |
6.10 |
1.34 |
4.18 |
6.43 |
0 |
2.16 |
4.59 |
1.34 |
3.01 |
| 25% MPL |
4.58 |
0 |
1.96 |
5.95 |
0 |
0.59 |
2.41 |
2.3 |
2.87 |
| NR assay |
100% MPL |
13.82 |
15.3 |
10.78 |
14.91 |
19.09 |
35.18 |
14.07 |
10.75 |
23.25 |
| 75% MPL |
7.79 |
9.24 |
3.79 |
13.57 |
8.79 |
22.36 |
9.80 |
15.23 |
0 |
| 50% MPL |
6.53 |
0.76 |
6.59 |
14.91 |
5.00 |
19.96 |
11.56 |
9.09 |
1.30 |
| 25% MPL |
6.37 |
0.46 |
4.19 |
5.19 |
5.23 |
10.38 |
3.18 |
6.97 |
0 |
| MPL: Maximal Permissible Levels; h: Hours; MTT: methyl-thiazol-tetrazolium; NR: Neutral Red. |
DISCUSSION
The present paper demonstrated the specific effects of selected concentrations of cadmium, nickel and lead on the green algae Scenedesmus incrassatulus and four human cell lines. Exposition to Cd2+, Ni2+ and Pb2+ led to a different influence on the growth and development of S. incrassatulus. Lead treatment inhibited the algal growth, exposition to cadmium-induced stimulatory effect, and neither of the tested nickel concentrations influenced the growth and development of algae. Data from existing reports discuss about the influence of heavy metals on species from the genus Scenedesmus which supported the observations of the study and suggested species specificity with respect to algal reaction to heavy metals. Mercury (Hg) in concentrations 2.5-5 mg/L inhibited completely the growth of Scenedesmus acutus.15 Cadmium caused 50% inhibition of Scenedesmus armatus growth after 24 h exposition to concentrations ranging from 0.46 to 0.54 mg/L.14 On the other hand, low concentrations of Cd and Pb induced stimulatory effects on the growth of Scenedesmus quadricauda, Scenedesmus pectinatus and Scenedesmus acuminatus.9,12,16
In some algal species, heavy metals influenced specific morphological characteristics. For example, Scenedesmus ernardii underwent morphological changes in response to Zn, Cd and Pb exposition: up to 82% dissociation to single cells after exposure to zinc, formation of unusual coenobia, extensive secretion of mucilage and clustering of cells and coenobia as a result of Cd and Pb treatment.11
The present study demonstrated the influence of three types of heavy metals on the morphology of Scenedesmus incrassatulus BOHLIN. The chosen test-object generated coenobia composed of 2, 4 or 8 cells. The inner cells in four- and eight-cellular coenobia acquired a serial, alternative or irregular orientation. The shape of the cells ranged from narrow to wide spindle-shaped, gradually thinning with sharp edges of the cells, bent to the center of the coenobium (more prominent in the outer cells compared to the inner ones). Intercellular connections spanned from 1/2 to 2/3 through the length of adjacent cells. The variation in the taxonomical characteristics of S. incrassatulus have been described previously.28 A stable taxonomical sign for the discrimination of S. incrassatulus from other closely related species was the “shape of peripheral cells”. Typical S. incrassatulus peripheral cells were spindle-shaped gradually thinning with pointed ends bent to the center of the coenobium. Except the shape of peripheral cells, the experiments conducted in the present study aimed to analyze two other taxonomical characteristics—number of cells in the coenobium/single cells and position of the inner cells in the coenobium.
Concerning the sign “cell number”, S. incrassatulus reacted to the presence of cadmium and nickel which supported the formation of single cells and reduced the coenobia composition. This effect was evident even after 24 h of treatment, at MPL concentrations and was more pronounced with cadmium treatment (Figure 2). Similar reaction of Scenedesmus incrassatulus after exposition to divalent copper and cadmium was detected.13 On the basis of related experiments, the divalent Cu and Cdinduced single cells commonly from four-cellular Scenedesmus incrassatulus, while being exposed to hexavalent chromium (Cr) led to the appearance of coenobia without sheath that corresponded to the formation of autospores.
MPL concentrations of cadmium and nickel affected also the position of inner cells in the coenobium by increasing the percent of coenobia with irregularly positioned cells and decreasing the composition of coenobia with linearly positioned cells. In the presence of nickel, these changes appeared already after 24 h. These observations demonstrated that the heavy metals Cd and Ni influence the development of S. incrassatulus even after a short exposition (24 h) by varying the characteristics “cell number” and “position of inner cells in the coenobium”. But these taxonomical traits can be influenced also by environmental factors and vary considerably. Therefore, these morphological reactions cannot serve as reliable markers specific to the presence of cadmium and nickel.
The morphology of peripheral cells of S. incrassatulus was influenced by the exposition to lead. The effect of lead was the strongest compared to the other two heavy metals. The peripheral cell type “incrassatulus” decreased already following 24 h of exposition and after 72 h at MPL concentration of Pb2+, typical for the species in which peripheral cells were not present. This finding supported the conclusion that Scenedesmus incrassatulus manifested highest sensitivity to lead exposition, which negatively influenced the algal growth and significantly changed the morphology of the species in concentrations corresponding to MPL. Based on these data, S. incrassatulus can be recommended as a test-object for the presence of lead in fresh water basins.
А general objective of the present study was to suggest new sensitive test-systems for biomonitoring of Cd, Ni and Pb pollution that react to MPL and even lower than MPL concentrations of the heavy metals. Therefore, in addition to Scenedesmus incrassatulus other test-objects were also examined. Three cancer cell lines (HeLa, A549, Caco-2) and a cell line (FL) derived from normal amniotic cells were used to evaluate the effect of Cd, Ni and Pb on human cell growth in vitro. These experiments aimed to investigate whether the heavy metals exert cytotoxic and growth inhibitory effects on different cell lines and determine the most sensitive cell type.
Different assays were performed for the evaluation of cytotoxic effects in vitro – МТТ assay, ХТТ assay, LDH assay, NR test, assays determining cell number and total protein content, etc. Several of these assays were compared and it was suggested that MTT and NR tests showed the highest sensitivity.29 This accounted for the reason to choose NR and MTT assays for the evaluation of in vitro growth inhibitory and cytotoxic effects of heavy metals.
Mammalian cell lines have been extensively used to study the mechanisms of heavy metal toxicity17,20,21,29 and data from in vitro cytotoxicity assays have been reported for the cell lines A549, HeLa and Caco-2 treated with different heavy metals and heavy metal compounds.17,25,30 However, to date, evaluation of the inhibitory effect of MPL and lower than MPL heavy metals concentrations have not been reported and little is known on the potential use of human cell lines for detection of heavy metals. The results led to the conclusion that all tested cell lines (A549, HeLa, FL and Caco-2) could be used for detection of cadmium, nickel and lead MPL concentrations after 72 h of exposition. Among the four cell lines, A549 showed almost equal sensitivity to all three heavy metals, while the other cell lines were more sensitive to cadmium relative to lead. Moreover, A549 demonstrated the highest percent inhibition and this strong response was evident even at 24 h exposition to either Cd2+, Ni2+ or Pb2+. Therefore, it was concluded that A549 cell line showed the highest sensitivity to MPL concentrations of the three heavy metals compared to HeLa, FL and Caco-2 cell lines.
CONCLUSION
The present study provided an insight for the potential application of new test-systems (algal culture of Scenedesmus incrassatulus and human cell lines) for biomonitoring of pollution with cadmium, nickel and lead. The reported data demonstrated that these heavy metals have different effects on the tested cells, and these effects were dose and time-dependent. The cadmium, nickel and lead influenced different morphological features of the algal system during the exposure. Based on the reaction of the algal culture by changing the evaluated morphological features some of the heavy metal pollutants could be detected very early, especially when the reaction was metal-specific. All four tested human cell lines may be used as test-systems for assessment of heavy metal toxicity or for biomonitoring of pollution with cadmium, nickel and lead. The observations of the present study demonstrated that the cell line A549 was the most sensitive for the tested heavy metals.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.










