INTRODUCTION
Presently, 25% of prescribed drugs worldwide are derived from plant sources in spite of the great progress and advancement of organic synthesis.1 Medicinal plants offer unlimited opportunities for the discovery of new drugs in biomedical field. Most of the natural products used in folk remedy have scientific evidences with regard to their biological activities. However, there is little evidence available concerning the possible toxicity those drugs or products from medicinal plants may cause to the consumers.2 The traditional use of any plant for medicinal purposes requires guarantees for the safety of such plant. Herbal medicines are usually complex mixtures of many bioactive compounds. Herbal medicines may differ from single-agent pharmaceuticals, phytomedicines due to the different mechanisms of action of their bioactive constituents.3 This is also reflected in their dose-response relationships and in synergistic/combinatorial effects. Pharmacological investigations have established their rising relevance in search of more reliable herbal drugs free of any side effects.4 Different types of interactions can arise whenever a chemical substance or drug administered to a biological system and a series of dose-related responses can occur. Thus, to identify potential health hazards before the drugs are administered to man, toxicity testing in animals is usually done on new drugs with doses well above the expected therapeutic range. Toxicity studies include a wide range of tests in different species with regular monitoring for biochemical or physiological anomalies seen in long-term administration of the drug.5
The types of toxicity studies which are routinely executed by pharmaceutical manufacturers in the search of a new drug include acute, sub-acute and chronic toxicity. Administration of a single dose or multiple doses in a period not beyond 24 hours, up to a limit of 2000 mg/kg produces acute toxicity. Objective of acute toxicity tests is to detect a dose producing major adverse effects and an assessment of the minimum dose causing lethality.6
Calotropis gigantea (Linn.) R. Br. of family Asclepiadaceae is a perennial shrub and widely distributed in tropical and subtropical region and most abundant in Bangladesh, India, Burma and Pakistan.7,8,9,10 The root, stem, leaves, flower and latex of C. gigantea are reported to be utilized in traditional medicine for the treatment of toothache, ear ache, eczema, syphilis, elephantiasis, injury, pain, ulceration, epilepsy, anxiety and mental disorders.11,12
Traditionally, the root of Calotropis gigantea is used in treatment of leprosy, asthma, bronchitis, and as an expectorant.13,14 Calotropis gigantea reported to exhibit free radical scavenging15,16 and pro-coagulation activity,17 pregnancy interceptive,18 anticancer,19 immunomodulatory,20 wound healing,21 anti-inflammatory22,23,24,25 and hepatoprotective16,26 activity.
The major phytochemicals of Calotropis gigantea are flavonol, glycoside, uscharidin, calotropin, frugoside, calotroposides A to G. Other constituents are α-amyrin, β-amyrin, taraxasterol, β-sitosterol, α– and β-amyrinmethylbutazone, gigantursenylacetate A and B. It’s latex rich in lupeol, calotropin, calotoxin and uscharchin.27 In current times there is a growing awareness and interest in medicinal plants and their preparations.28 Lack of scientific and clinical data in support of better understanding of the efficacy and safety of the drugs, is the major difficulty to the use of traditional herbal preparations.
The sub-acute toxicity data may be required to envisage the safety and effects of long term exposure to a specific medicinal plant. This study therefore was carried out to evaluate the sub-acute toxic effects of the ethanol and water extract of Calotropis gigantea latex in mice.
MATERIALS AND METHODS
Collection and Extraction of Plant Material
C. gigantea plant was collected from the out fields of Vidyasagar University, Midnapore, West Bengal, India and authenticated from Botanical Survey of India (BSI), Ministry of Environment and Forest (MoEF), Government of India, Howrah (Identification No. CNH/2014/Tech.II/55).
The fresh latex of C. gigantea was collected from the aerial parts of mature plants and was then spread into petri dishes for air dry in room temperature under shade up to 4 to 5 days. Then 250 g of dried latex was subjected for size reduction to coarse powder. Then in 450 ml of ethanol and water, powdered latex was dissolved separately and was incubated for 48 hours in room temperature and was filtered using Whatmann filter paper. The filtrates were dried in EYELA CCA 1110 rotary evaporator to produce ethanol extracts of Calotropis gigantea latex (EECGL) and water extracts of Calotropis gigantea latex (WECGL) extracts (yields 4.5% and 2.8% respectively).29 Dried extracts were stored in air tight containers at 4°C till further use.
Animals
Healthy male Swiss albino mice (20-25 g) were selected for toxicity test. The mice were grouped and housed in poly acrylic cage (38×23×10 cm) with 6 animals per cage. The animals were kept on a 12 h light: 12 h dark regime at 25°C for 7 days before commencement of the experiment. The animals had free access to standard diet and water.30 Mice were deprived of food but not water prior to administration of the test extracts. The study was approved by the Institutional Animal Ethical Committee (IAEC), registered under Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment, Forests and Climate Change (MoEFCC), Government of India and performed in compliance with the relevant laws and guidelines of the CPCSEA.
Treatment Schedule
The sub-acute toxicity study on the above mentioned plant extracts was performed as per the Organisation for Economic Co-operation and Development (OECD) guidelines 40731 with slight modifications, where 2000 mg/kg was used as the limit test dose. The ethanol and water extracts of Calotropis gigantea latex were administered intraperitoneally prior up to the bladder32 at the dose levels of 50, 100, 200, 500, 1000 and 2000 mg/kg body weight to the group II to VI animals respectively and distilled water to the control group (Group I) animals by sterile syringe daily for 28 days.33,34 Toxicity studies were conducted as per internationally accepted protocol in Swiss albino mice. The animals were divided into 6 groups containing 6 animals each. The experimental design was given below:
Group I : Control
Group II : EECGL /WECGL (50 mg/kg body weight)
Group III : EECGL/WECGL (100 mg/kg body weight)
Group IV : EECGL/WECGL (200 mg/kg body weight)
Group V : EECGL/WECGL (500 mg/kg body weight)
Group VI : EECGL/WECGL (1000 mg/kg body weight)
Group VII : EECGL/WECGL (2000 mg/kg body weight)
The feed and volume of water consumed by mice in each group were monitored daily. Weights of the mice in all the groups were recorded daily during the treatment schedule and on the last day of study. Doses of the extract administered were adjusted accordingly. On the 29th day of the experiment, the mice were anesthetized with pentobarbital sodium (50 mg/kg, i/p) and sacrificed by cervical dislocation. The test organs of the animals were opened up surgically and blood samples were collected by cardiac puncture.
Blood Sample Collection
On the last day of the experiment, 2 sets of blood samples were collected from all the animals via cardiac puncture using a 5 ml sterile syringe. Two ml of blood sample was collected into sterile container containing ethylenediaminetetraacetic acid (EDTA) as anticoagulant for the determination of haematological parameters. For serum analysis blood samples were collected into another anticoagulant free sterile container, allowed to stand at room temperature and centrifuged at 2000 rpm for 10 minutes. The supernatants were then collected and stored at -20ºC for biochemical analysis.
Organ Weight
On 29th day, all the animals were sacrificed. Heart, liver, lungs, spleen, and kidneys were carefully dissected out and weighed.
Haematological Analysis
Red blood cell (RBC) count: Blood was diluted with RBC dilution fluid (1:200). Total erythrocytes were counted in the Neubaur haemocytometer chamber.35
White blood cell (WBC) count: Blood was diluted with white blood cell (WBC) dilution fluid (1:20) and loaded in Neubaur haemocytometer chamber. Four large (1 sq mm) corner squares of the haemocytometer chamber were counted under the microscope.35
Determination of haemoglobin: At first 20 μl of blood was transferred into a test tube containing 5 ml of Drabkin’s solution. After adjusting the photoelectric colorimeter at 540 nm with a blank the OD of sample was read.36
Estimation of Urea
Urea was determined by the modified method of Natelson et al.37,38 To 0.1 ml of serum, 3.3 ml of water, 0.3 ml each of 10% sodium tungstate and 0.67 N sulphuric acid were added. The suspensions were centrifuged and to 1.0 ml of the supernatant, 1.0 ml of water, 0.4 ml of diacetylmonoxime and 2.6 ml of 0.67 N sulphuric acid-phosphoric acid reagents were added. Standard urea (20 to 50 µg/ml) were also treated in a similar manner and all the tubes were heated in a boiling water bath for 30 minutes, cooled and the color developed was measured at 480 nm in a Shimadzu spectrophotometer (UV-Shimadzu-245, Japan). The values were expressed as mg of urea/dl of blood.
Estimation of Creatinine
Creatinine was described according to the modified method of Brod and Sirota.39,40 At first, protein free filtrate was prepared. One ml of serum was precipitated with 8.0 ml of water, 0.5 ml of 2/3 N sulphuric acid and 0.5 ml of 40% sodium tunsgstate. After that, 5.0 ml clear filtrate was taken and 1.5 ml of saturated picric acid and 1.5 ml of 0.75 N sodium hydroxide were added to it. Standard and blank were also prepared similarly. The color intensity was measured at 530 nm in a Shimadzu spectrophotometer (UV-Shimadzu-245, Japan). The values were expressed as mg of creatinine/dl of blood.
Measurement of Serum Alkaline Phosphate (ALP)
Activity of serum alkaline phosphatase were estimated by taking 0.25 mL of serum in a centrifuge tube containing 1 ml buffer (1 mM of p-nitrophenol phosphate in 1 M Tris buffer, pH 8.0); the mixture was then subjected to be incubated at 37ºC for 30 minutes in a water bath. The activity was measured spectrophtometrically at 420 nm.41
Measurement of Serum Glutamate Oxaloacetate Transaminase (SGOT) and Serum Glutamate Pyruvate Transaminase (SGPT)
For the estimation of SGOT, to 1.0 ml of the buffered substrate (200 mM/L of DL-aspartate and 2 mM/L of α-ketoglutarate, pH=7.4), 0.1 ml of serum was added and incubated at 37°C for one hour. Then 1 ml of dinitrophenyl hydrazine (DNPH) was added and kept for 20 minutes at room temperature. After 20 minutes, 10 ml of 0.4 N sodium hydroxide was added and the color intensity was measured at 505-540 nm in a Shimadzu spectrophotometer (UV-Shimadzu-245, Japan) after 10 minutes against the reagent blank. The standard and blank were also prepared by the same process. The enzyme activity in serum was expressed as IU/lit.
For the estimation of SGPT, to 1.0 ml of the buffered substrate (200 mM/L of DL-alanine and 2 mM/L of α-ketoglutarate pH=7.4), 0.1 ml of serum was added and incubated at 37°C for on hour. The reaction was arrested by the addition of 1.0 ml of dinitrophenyl hydrazine and left aside for 20 minutes at room temperature. The color developed by the addition of 10 ml of 0.4 N sodium hydroxide was read at 505-540 nm in a Shimadzu spectrophotometer (UV-Shimadzu-245, Japan) against the reagent blank. The enzyme activity in serum was expressed as IU/lit.42
Estimation of Blood Glucose
Blood glucose was measured according to the method of Nelson-Somogyi.43,44 Somogyi’s copper reagent was prepared by dissolving 24 g anhydrous sodium carbonate and 12 g of sodium potassium tartrate in about 250 ml of distilled water. To this 4 g copper sulphate as a 10% (w/v) solution was added and mixed followed by the addition of 16 g of sodium bicarbonate. Then 180 g of sodium sulphate was dissolved in about 500 ml of distilled water and boiled to expel air. After cooling, the two solutions were mixed and the volume was made up to 1000 ml. Nelson’s arsenomolybdate reagent was prepared by dissolving 25 g ammonium heptamolybdate in 450 ml of water. Then 21 ml of sulphuric acid was added and mixed well. To the mixture 3.0 g disodium hydrogen arsenate dissolved in 25 ml of distilled water was added. The solution was mixed well and incubated for 24 hours at 37 °C. From the sample, one ml of aliquot was pipetted out. To this 1.0 ml of Somogyi’s copper reagent was added. The mixture was then placed in a bath of boiling water and heated for 20 minutes. After cooling under tap water 1.0 ml of Nelson’s arsenomolybdate reagent was added with immediate mixing till the effervescence ceased. The intensity of color was measured spectrophtometrically at 540 nm.
Measurement of Serum Cholesterol
At first 0.1 ml of serum was mixed with 6 ml of glacial acetic acid. After addition of 4 ml of color reagent (1 ml of 10% FeCl3, 6 H2O, 15 ml of conc. H2SO4) it was mixed vigorously and allowed to stand for 20 min. The reading was taken at 570 nm.45 The amount of cholesterol present is calculated by plotting the standard curve.
Measurement of Serum Total Protein
Different dilutions of BSA solutions are prepared by mixing stock BSA solution (1 mg/ml) and water. The final volume in each of the test tubes is 6 ml. The BSA range is 0.05 to 1 mg/ml. From these different dilutions, protein solutions were pipetted out to different test tubes and 5 ml of alkaline copper sulphate reagent were added. The solutions were mixed well. Then 0.5 ml of reagent Folin-Ciocalteau solution (reagent solutions) was added to each tube and incubated at 37°C for 30 min. The optical density was measured at 660 nm.46 The absorbance was plotted against protein concentration to get a standard calibration curve.
Histopathology Analysis
All the animals from each group were sacrificed for histopathological examinations of major internal organs. Organs such as liver, kidney were collected from all the animals. The collected organs were weighed and preserved in 10% neutral buffered formalin, then dehydrated in alcohols and embedded in paraffin. Five micron thickness of tissue sections were stained with haematoxylin and eosin (H and E) for histopathological study.
Statistical Analysis
All the parameters were performed in triplicate manner. The data was expressed as Mean±SEM, comparisons between control and treated groups were analyzed by using the one-way analysis of variance (ANOVA) considering p<0.05 as a limit of significance.
RESULTS
Relative Organ Weights (ROW) of Male Mice
Relative organ weights of 28-days treated mice are shown in Tables 1A and 1B. The relative organ weights recorded in the treatment groups did not show any significant difference (p<0.05) up to the dose level of 1000 mg/kg body wt. compared to the control.
| Table 1A: Effect of water extract of Calotropis gigantea latex (WECGL) administration for 28 days on body and different organ weight in Swiss albino mice. |
| Weight |
Control |
50 mg/kg body wt. |
100 mg/kg body wt. |
200 mg/kg body wt. |
500 mg/kg body wt. |
1000 mg/kg body wt. |
2000 mg/kg body wt. |
| Initial body weight (gm) |
27.66±0.33 |
27±0.57 |
27.7±0.33 |
27.88±0.57 |
28±0.57 |
28±0.57 |
28±0.57 |
| Final body weight (gm) |
31±0.57 |
31±0.57 |
31±0.57 |
31±0.57 |
32±0.57 |
32±0.57 |
35±0.57* |
| Weight of heart (gm) |
0.22±.005 |
0.22±.005 |
0.22±.005 |
0.21±.005 |
0.21±.003 |
0.2±.003 |
0.19±.005* |
| Weight of spleen (gm) |
0.24±.008 |
0.24±.008 |
0.26±.005 |
0.27±.003 |
0.29±.005 |
0.30±.008 |
0.32±.011* |
| Weight of liver (gm) |
0.33±.008 |
0.33±.008 |
0.34±.008 |
0.36±.005 |
0.36±.008 |
0.41±.008 |
0.41±.011* |
| Weight of kidney(gm) |
0.38±.003 |
0.36±.005 |
0.36±.005 |
0.35±.005 |
0.35±.005 |
0.32±.003 |
0.3±.005* |
| Results are expressed as Mean ± SEM, Analysis is done by one way ANOVA. Comparison was done between control group versus all other groups (*indicates p<0.05). |
| Table 1B: Effect of ethanolic extract of Calotropis gigantea latex (EECGL) administration for 28 days on body and different organ weight in Swiss albino mice. |
| Weight |
Control |
50 mg/kg body wt. |
100 mg/kg body wt. |
200 mg/kg body wt. |
500 mg/kg body wt. |
1000 mg/kg body wt. |
2000 mg/kg body wt. |
| Initial body weight (gm) |
27±0.57 |
27±0.57 |
27.33±0.33 |
28±0.57 |
28±0.57 |
28.66±0.88 |
29±0.57 |
| Final body weight (gm) |
31.33±0.66 |
31±0.57 |
31±.57 |
33±0.57 |
34±0.57 |
34.66±0.88 |
35±0.57 |
| Weight of heart (gm) |
0.23±.005 |
0.22±.005 |
0.22±.005 |
0.22±.008 |
0.2±.005 |
0.2±.008 |
0.19±.005* |
| Weight of spleen (gm) |
0.27±.005 |
0.27±.003 |
0.27±.005 |
0.28±.005 |
0.29±.005 |
0.3±.005 |
0.32±.008* |
| Weight of liver (gm) |
0.31±.005 |
0.32±.005 |
0.33±.005 |
0.32±.005 |
0.32±.011 |
0.33±.005 |
0.35±.015* |
| Weight of kidney(gm) |
0.39±.005 |
0.36±.008 |
0.36±.005 |
0.35±.005 |
0.35±.005 |
0.33±.003 |
0.31±.005* |
| Results are expressed as Mean±SEM; n=6. Analysis is done by one way ANOVA. Comparison was done between control group versus all other groups (*indicates p<0.05). |
Haematological Parameters Analysis
The effects of C. gigantea latex extracts at sub-acute doses on haematological parameters are presented in Tables 2A and 2B. Most haematological parameters (haemoglobin, total RBC count, total WBC count) in treated mice were not significantly different from the control group animals, with the exception of marginal variations in the parameters. No significant toxicity seen up to the dose level of 1000 mg/kg body wt. in haemoglobin percentage and total WBC count in latex extracts treated mice than that of the control animals. There was no trace of toxicity in total RBC counts in latex extracts treated mice up to the dose level of 2000 mg/kg body wt.
| Table 2A: Haematological parameters after WECGL administration for 28 days in Swiss albino mice. |
| Haematological parameters |
Control |
50 mg/kg body wt. |
100 mg/kg body wt. |
200 mg/kg body wt. |
500 mg/kg body wt. |
1000 mg/kg body wt. |
2000 mg/kg body wt. |
| Haemoglobin (%) |
15±0.577 |
15±0.55 |
14.8±0.25 |
14.5±0.4 |
14.5±0.54 |
14±0.55 |
13.5±0.54* |
| Total RBC count (cu.mm) |
6±0.145 |
6±0.012 |
5.8±0.104 |
5.8±0.05 |
5.5±0.057 |
5.1±0.04 |
5.1±0.02 |
| Total WBC count (cu.mm) |
5100±58 |
5100±50 |
5200±62 |
5300±60 |
5300±78 |
5400±65 |
5560±70* |
| Results are expressed as Mean ± SEM; n=6. Analysis is done by one way ANOVA. Comparison was done between control group versus all other groups (*indicates p<0.05). |
| Table 2B: Haematological parameters after EECGL administration for 28 days in Swiss albino mice. |
| Haematological parameters |
Control |
50 mg/kg body wt. |
100 mg/kg body wt. |
200 mg/kg body wt. |
500 mg/kg body wt. |
1000 mg/kg body wt. |
2000 mg/kg body wt. |
| Haemoglobin (%) |
15±0.577 |
15±0.52 |
14.54±0.25 |
14.5±0.43 |
14.5±0.56 |
14±0.55 |
13.8±0.64* |
| Total RBC count ( cu.mm) |
6±0.145 |
6±0.109 |
5.5±0.124 |
5.5±0.057 |
5.6±0.057 |
5.2±0.042 |
5.2±0.022 |
| Total WBC count (cu.mm) |
5100±58 |
5100±52 |
5300±82 |
5345±80 |
5400±88 |
5545±85 |
5550±80* |
| Results are expressed as Mean±SEM; n=6. Analysis is done by one way ANOVA. Comparison was done between control group versus all other groups (*indicates p<0.05). |
Effect of EECGL and WECGL on Hepatic and Renal Biomarkers of Mice
The results of hepatic and renal biomarkers following the treatment of C. gigantea latex extracts in mice are shown on Figures 1 to 8.
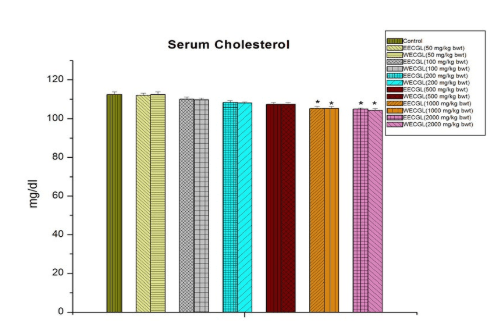
No significant sub-acute toxicity was seen in the level of urea (Figure 1), creatinine (Figure 2), alkaline phosphotase (Figure 3), and serum cholesterol (Figure 7) in mice treated with EECGL and WECGL up to the dose level of 500 mg/kg body wt./day for 28 days when compared with the control animals.
Figure 1: Shows serum urea level after treatment of EECGL and WECGL for 28 days in Swiss albino mice. Results are expressed as Mean±SEM; n=6. Analysis is done by one way ANOVA. Comparison was done between control group versus all other groups (*indicates p<0.05).
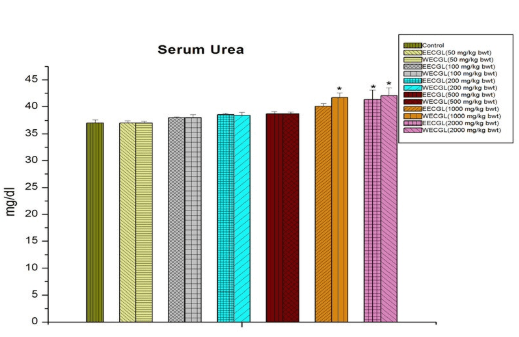
Figure 2: Serum creatinine after treatment of EECGL and WECGL for 28 days in Swiss albino mice. Results are expressed as Mean±SEM; n=6. Analysis is done by one way ANOVA. Comparison was done between control group versus all other groups (*indicates p<0.05).
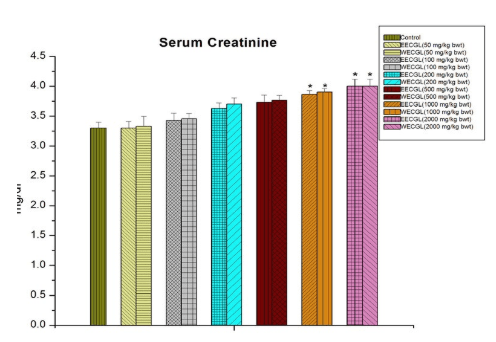
Figure 3: Shows serum ALP after treatment of EECGL and WECGL for 28 days in Swiss albino mice. Results are expressed as Mean±SEM; n=6. Analysis is done by one way ANOVA. Comparison was done between control group versus all other groups (*indicates p<0.05).
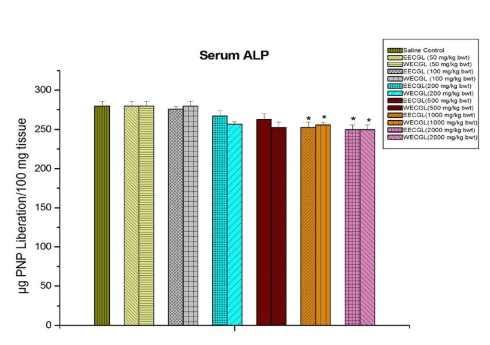
Figure 7: The diagram shows the measurement of serum cholesterol after treatment of EECGL and WECGL for 28 days in Swiss albino mice. Results are expressed as Mean±SEM; n=6. Analysis is done by one way ANOVA. Comparison was done between
control group versus all other groups (*indicates p<0.05).
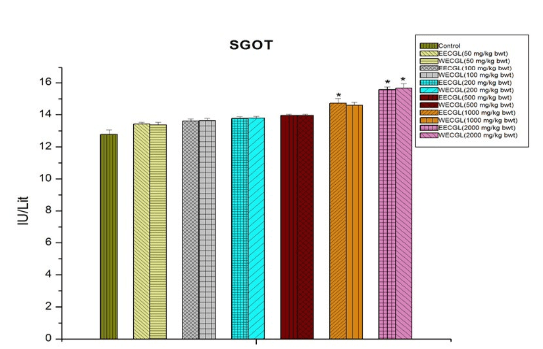
In case of blood glucose (Figure 6), SGPT (Figure 5) and serum total protein (Figure 8), significant sub-acute toxicity was not observed up to the dose level of 1000 mg/kg body wt./day.
Figure 5: Level of SGPT after treatment of EECGL and WECGL for 28 days in Swiss albino mice. Results are expressed as Mean±SEM; n=6. Analysis is done by one way ANOVA. Comparison was done between control group versus all other groups (*indicates p<0.05).
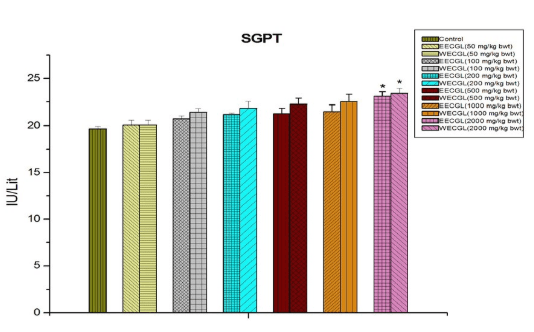
Figure 6: Shows the blood glucose level after treatment of EECGL and WECGL for 28 days in Swiss albino mice. Results are expressed as Mean±SEM; n=6. Analysis is done by one way ANOVA. Comparison was done between control group versus all other groups (*indicates p<0.05).
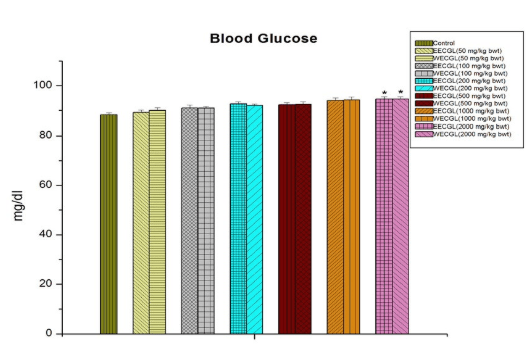
Figure 8: Shows serum total protein after treatment of EECGL and WECGL for 28 days in Swiss albino mice. Results are expressed as Mean±SEM; n=6. Analysis is done by one way ANOVA. Comparison was done between control group versus all other groups (*indicates p<0.05)
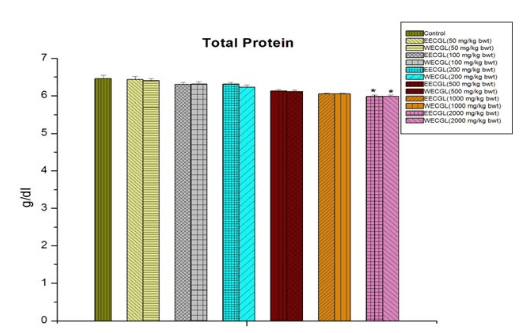
WECGL produces no toxicity in case of SGOT (Figure 4) up to 1000 mg/kg body wt./day but EECGL causes significant (p<0.05) toxicity at this dose level.
Figure 4: The effect of SGOT after treatment of EECGL and WECGL for 28 days in Swiss albino mice. Results are expressed as Mean±SEM; n=6. Analysis is done by one way ANOVA. Comparison was done between control group versus all other groups (*indicates p<0.05).
Histopathological Study
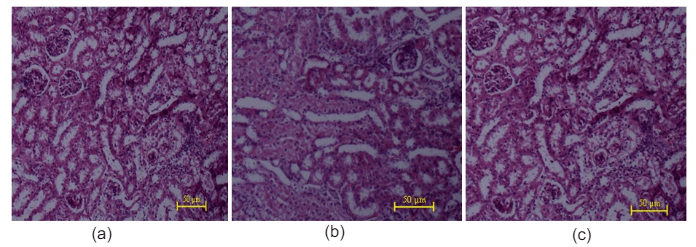
Histopathological studies of the liver and kidney tissues in the control and the C. gigantea extract treated groups showed no differences, indicating that treatment of EECGL and WECGL did not result in any adverse or abnormal toxicological effect on these vital organs (Figures 9, 10, 11 and 12).
Figure 9: Histopathological studies of liver tissue: (a) Control mice, (b) EECGL treated (50 mg/kg body wt.), (c) EECGL treated (2000 mg/kg body wt.).
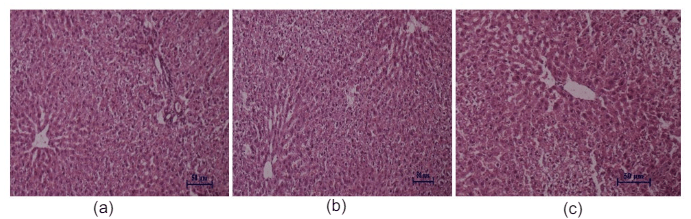
Figure 10: Histopathological studies of liver tissue: (a) Control mice, (b) WECGL treated (50 mg/kg body wt.), (c) WECGL treated (2000 mg/kg body wt.).
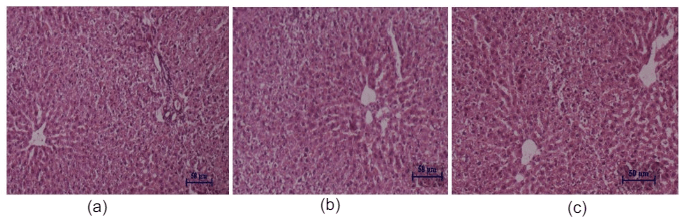
Figure 11: Histopathological studies of kidney tissue: (a) Control mice, (b) EECGL treated (50 mg/kg body wt.), (c) EECGL treated (2000 mg/kg body wt.).
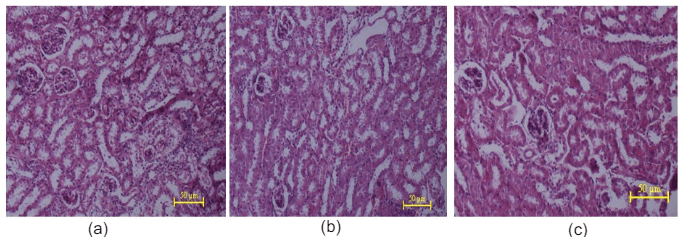
Figure 12: Histopathological studies of kidney tissue: (a) Control mice, (b) WECGL treated (50 mg/kg body wt.), (c) WECGL treated (2000 mg/kg body wt.).
DISCUSSION
For centuries, natural products especially medicinal plants are used for the treatment of different diseases.47 In the screening of pharmacological efficacy of a medicinal plant, the initial step is the assessment and evaluation of its toxic characteristics and the present study was undertaken to investigate the sub-acute toxicity of C. gigantea in a mammalian model.
No significant changes was observed in the weights of the heart, liver, spleen, kidneys suggesting that the administration of C. gigantea extract at the sub-acute doses had no effect on the normal growth. In toxicity studies, the worth of weighing organs takes account of their sensitivity to envisage toxicity, enzyme stimulation, physiologic perturbations, and acute injury.48 The relative organs weights are also relatively sensitive indicators for particular organs in toxicity studies.49 The findings of this study exposed that the vital organs, such as liver, kidneys, were not adversely affected for toxicity throughout the treatment. Since there was no reduction in body and relative organ weights of the treated animals at any of the tested dose level, we concluded that the extracts are nontoxic to the analyzed organs.
To evaluate the probable changes in hepatic and renal functions influenced by the extracts the serum haematology and clinical biochemistry studies were done liver and kidney function analysis is very important in the toxicity evaluation of plant extracts as they are both necessary for the survival of an organism.50 The assessment of activities of serum marker enzymes plays important role in the evaluation of plant extract for its toxicity risk. The enzymes considered in this study, are valuable marker enzymes of liver cytolysis and liver cell membrane damage. SGOT and SGPT, the transaminases are well known good indicators of liver function and used as biomarkers to conclude the probable toxicity of drugs. Normally, destruction to the liver parenchymal cells will result in an increase of both these enzymes in the blood.51 Alkaline phosphatase is a marker enzyme for the plasma membrane and endoplasmic reticulum of the tissue studied. High levels of alkaline phosphatase are reported in liver diseases or hepatotoxicity.52 The insignificant changes in alkaline phosphatase in male mice at all dose level suggest that acute administration of C. gigantea extract does not affect the hepatocyte function in mice. Renal dysfunction can be measured by simultaneous measurements of urea, creatinine and their normal levels observed at reduced renal problems.53 Higher than normal levels of serum creatinine and urea are good indicators of renal function abnormality.54 Thus, the decrease in serum creatinine concentrations with concomitant decrease in the serum urea concentration in the treated rats suggests that functioning of the kidney is normal. In the present study, changes in serum urea, creatinine levels in C. gigantea extract treated groups showed non-significant differences indicating a normal renal function up to the dose level of 500 mg/kg body wt.
Evaluation of haematological parameters can be used to estimate the extent of the harmful effect of C. gigantea extract on the blood of an animal. It can also be used to explain blood related functions of a plant extract or its products.55 Moreover, for risk evaluation such analysis is important as haematological changes have higher prognostic value for human toxicity when the data are obtained from mammalian studies.56 A haemogram was undertaken for all the C. gigantea extract treated and control groups and the results show no significant effects up to the dose level of 1000 mg/kg bw. The non-significant effect of the extract on total red blood cells, Hb percentage indicates that the C. gigantea extract does not affect the RBC morphology or formation, or its osmotic fragility.57 Leukocytes are the first line of cellular defence that counter tissue injury, infectious agents or inflammatory process. Furthermore, C. gigantea extracts produced no significant changes in WBC count, which further confirmed the above findings. A normal haematological profile of C. gigantea extract treated groups also further justified the non-toxic nature of C. gigantea extract. In histopathological studies, the liver of treated animals showed normal histological feature at 50, 2000 mg/kg. No degeneration of hepatocyte, focal steatosis, congestion of central vein and inflammation of portal tract when compared with control animals. The kidney of treated rats showed normal glomeruli and there is no necrosis of tubular epithelium in the kidney. Gross histological examination of liver and kidney on did not reveal any abnormalities. Thus, it was concluded that C. gigantea did not produce any toxic effect in male albino mice.
From these findings, we may conclude that EECGL and WECGL are not toxic up to the dose level of 500 mg/kg body wt./day for 28 days and did not produce any noteworthy significant (p<0.05) alterations in relative organ weights, haematological, hepatic and renal biomarkers except blood glucose, serum protein and serum glutamate pyruvate transaminase (SGPT). No significant toxicity was seen up to the dose level of 1000 mg/kg body wt./day for 28 days in case of blood glucose, serum protein and SGPT, which are important toxicity biomarkers. As there were no significant adverse effects on the liver and kidney histology, haematological and serum biomarkers up to the dose level of 500 mg/kg body wt., it may be concluded that the ethanolic and water extract of C .gigantea latex did not induce any damage to the vital organs and may be quite safe for mammals.
CONCLUSION
In conclusion, the present investigation establishes that the ethanolic extract and water extract of C. gigantea latex may be considered as absolutely safe up to 500 mg/kg body wt. dose level, as it did not create any toxicological effects on selected body organs, haematological and serum biomarkers of mice during the sub-acute toxicity study. So, the findings suggest that ethanolic and water extracts of Calotropis gigantea latex do not produce any effective sub-acute toxicity in mice and may be considered as phytomedicinal therapeutic agents.
ACKNOWLEDGEMENT
The authors are grateful to authority of Vidyasagar University, Midnapore, India for providing all the facilities to execute this study.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.

















