INTRODUCTION
Pesticides are used worldwide to control both agricultural and household pests. In 2001, United States used approximately 122 million pounds of insecticides, and 12% of those compounds were for home and garden use.1 One of the most frequently used classes of pesticides is the pyrethroids,2 the synthetic analogs of the naturally occurring toxin pyrethrin, which is derived from the flowers of Chrysanthemum cinerariafolium. They reported approximately one fourth of the worldwide market for insecticides in 1998, and their use is continually growing.3 Pyrethroids exhibit their toxic action by modifying the “gating” characteristics of neuronal voltage-sensitive sodium channels to delay their closure, thereby prolonging neuronal excitation.4,5 Pyrethroids are considerably less toxic to mammals than organochlorines, organophosphates and carbamates. Cypermethrin is a synthetic pyrethroid used not only as ectoparasiticide in animals but also used widely as an insecticide in agriculture and public health programmes. In recent years, much attention is being focused on the possible role of essential trace elements in providing the necessary preventive efficacy with least toxicity and side effects.6,7,8 Zinc, a key constituent or cofactor of many mammalian proteins, is intensively being studied for its protective efficiency in various models of animal toxicity.8 A number of studies have strongly suggested zinc to be a beneficial agent in mitigating the damage arising in the setting of increased oxidative stress.8,9,10
The reproductive functions are maintained in vertebrates by hormones of the hypothalamic-pituitary-gonadal axis. Steroids from the gonads are influenced by the hypothalamic-pituitary (HP) axis and send feedback signals to the HP axis. Puberty can be divided into central and the peripheral puberty. Central puberty involves the onset of GnRH and gonadotropin secretions as the HP axis matures. The gonads are stimulated, the HP axis induces gonadal secretion of sex steroid hormones, and the steroids then send feedback to the HP axis. Peripheral puberty includes the processes other than that of hypothalamic-pituitary-gonadal axis. In the female, secondary sex characteristics such as the development of mammary glands, the vaginal opening, and uterine hypertrophy11 are included under this peripheral puberty. Though the onset of puberty is a genetically driven event,12 it can be changed by environmental factors,13 nutritional states, metabolic status, phytoestrogens14 and pesticides.15
The present study was aimed to investigate cypermethrin mediated changes in reproductive functions and also to find out the attenuating role of zinc on cypermethrin induced reproductive toxicity in female prepubertal rat.
MATERIALS AND METHODS
Chemicals and Reagents
A commercial formulation of cypermethrin [(RS)-α -cyano-3-phenoxybenzyl (1RS)-cis-trans-3-(2, 2-dichlorovinyl) 2,2-dimethylcyclopropanecarboxylate] 10% emulsifiable concentrate (EC), named ‘‘Ustad’’ (United Phosphorus Limited, Hyderabad, TS, India) was used in the experiments. Nicotinamide adenine dinucleotide (NAD), dehydroepiandrosterone sulphate, nicotinamide adenine dinucleotide phosphate, glucose- 6-phosphate, bovine serum albumin were purchased from Sigma Aldrich Inc., USA, LH ELISA Kit (Catalog No. CSB-E12654r), FSH ELISA Kit (Catalog No. CSB-E06869r), Estradiol ELISA Kit (Catalog No. CAYMAN 582251) were used all other chemicals used were purchased from Himedia India Ltd., Merck India Ltd., etc.
Animal Care and Treatment
Sixty-six Wistar female prepubertal rats at 25 to 30 days of age (weighing 50 to 60 g) were taken and acclimatized for 10 days before the start of the experimental procedure. The animals were housed in labelled cages with solid plastic sides and stainless-steel grid tops and floors, in a room designed for control of temperature (approximately 25±2 °C), and light cycle (12 h light, 12 h dark). Animals were fed standard laboratory pellets diet and water ad libitum. The experiment was conducted strictly in accordance to the Institution’s Animal Ethical Committee. After 10 days of acclimatization, the animals were randomly divided to the control and experimental groups, each containing 11 rats. Groups were designed as:
1. Group I: Control (5 ml/kg body wt.)
2. Group II: Zinc (227 mg/l in drinking water) control
3. Group III: Cypermethrin-treated (34.33 mg/kg body wt., Low dose) group
4. Group IV: Zinc+Cypermethrin-treated (34.33 mg/kg body wt., Low dose) group
5. Group V: Cypermethrin-treated (51.5 mg/kg body wt., High dose) group
6. Group VI: Zinc+Cypermethrin-treated (51.5 mg/kg body wt., High dose) group
A commercial formulation of cypermethrin 10% Emulsifiable Concentrate (EC) was used in this study. Adequate dilutions were done with distilled water to get test concentrations (34.33 and 51.5 mg/kg body wt.). Solutions were freshly prepared immediately before usage. The doses were selected on the basis of acute toxicity dose of cypermethrin.16 Acute oral LD50 of female rat (albino) is 309 mg/kg body wt. We have considered the 1/9th LD50 and 1/6th LD50 doses from our dose selection study. Control rats received 5 ml of distilled water /kg body wt.
Body weights of the rats in each group were taken before and after the treatment period. All rats were euthanized 24 hours after the last dose. Five rats of each group were kept for the study of the age of vaginal opening and appearance of the first estrous.
Study of Puberty Onset and Estrous Cycle
To determine the onset of puberty for nine days from postnatal day 25, the vaginal opening of female prepubertal rats in each group was checked at 10 AM daily. The estrous cycle was examined daily by the modified method of Marcondeset et al17 with slight modifications and identified under a microscope (×100) using a vaginal smear flushed with physiological saline for 20 days from vaginal opening.
Measurement of Ovarian and Uterine Indices
Ovaries, uterus and adrenal glands were dissected out from each rat immediately after sacrifice and were done free from adherent tissue and the organ-weights were recorded.18 The ovaries and adrenal glands were taken out for the estimation of cholesterol, ∆5,3β-HSD, 17β-Hydroxysteroid dehydrogenase and oxidative stress parameters. Organ weight was expressed as organ index.
Ovarian index=(ovarian wt. (g))/(body wt. (g)) × 100
Uterine index=(uterus wt. (g))/ (body wt. (g)) × 100
Estimation of Ovarian and Adrenal Cholesterol
Ovary and adrenal gland of each rat were homogenized in 0.5% FeCl3 solution (20 mg/ml) for the estimation of cholesterol. The homogenate was then centrifuged at 2000 rpm for 10 min. Then 0.1 ml of the supernatant was mixed with 6 ml of glacial acetic acid.19 After addition of 4 ml of color reagent (1 ml of 10% FeCl3, 6 H2O, 15 ml of conc. H2SO4) it was mixed vigorously and allowed to stand for 20 min. The reading was taken at 570 nm. The amount of cholesterol present is calculated by plotting the standard curve.
Measurement of Ovarian and Adrenal Ascorbic Acid
For the estimation of ascorbic acid, the ovarian and adrenal tissues were homogenized using 2.5 ml of 5% metaphosphoric acid-10% acetic acid solutions. The mixture was centrifuged after extraction and a very small drop of concentrated bromine was added to the supernatant.20 Tube was shaken and kept for 10 min for complete oxidation. Excess liquid bromine was then removed. 0.5 ml of dinitrophenylhydrazine-thiourea reagent (2.2% 2, 4-DNPH in of 10N H2SO4, 5%) was added with 2 ml of tissue extract and incubated at 37 °C for 3 h and then 2.5 ml of 85% H2SO4 was slowly added in ice-cool condition. It was mixed well for half an hour in room temperature for color development and optical density was observed at 540 nm.
Ovarian and Adrenal ∆5,3β-HSD and 17β-HSD Activity
The tissues (ovary and adrenal gland) were homogenized carefully at 4 °C in 20% glycerol containing 0.01 M EDTA in 0.05 M phosphate buffer.21 Then the mixture was centrifuged at 10,000 rpm for 30 min at 4 °C. The supernatant (1 ml) was mixed with 1 ml of sodium pyrophosphate buffer (pH-8), 40 µl of dehydroepiandrosterone (DHEA) and finally after the addition of 0.1 ml of NAD, the activity ∆5,3β-HSD was measured at 340 nm against a blank containing no NAD.
One ml supernatant was added with 1 ml of 440 μM sodium pyrophosphate buffer, 960 µl of bovine serum albumin and 40 µl of ethanol containing testosterone. The activity of 17β-HSD was measured after the addition of NAD to the tissue supernatant mixture in a spectrophotometer at 340 nm against a blank (without NAD). One unit of enzyme activity was the amount causing a change in absorbance of 0.001 per minute at 340 nm.
Estimation of Luteinizing Hormone (LH), Follicle-Stimulating Hormone (FSH) and Estrogen
For the quantitative determination of luteinizing hormone, follicle-stimulating hormone and estradiol ELISA Kit (Catalog No. CSB-E12654r), ELISA Kit (Catalog No. CSB-E06869r) and ELISA kit (Catalog No. CAYMAN 582251) were used respectively. Assays were done according to the instruction of the manufacturers.
Estimation of Oxidative Stress Parameters
Ovarian Malondialdehyde (MDA)
Briefly 1 ml of ovarian homogenate (20 mg/ml) was mixed with 0.2 ml of 8.1 % sodium dodecyl sulfate, 1.5 ml of acetate buffer (20%, pH-3.5) and 1.5 ml of aqueous solution of (0.8%) thiobarbituric acid.23 After heating the mixture at 95 ˚C for 60 min, obtained red pigment was extracted with 5 ml of n-butanol-pyridine (15:1). Then it was centrifuged at 5000 rpm for 10 min at room temperature and the absorbance of supernatants was noted at 535 nm in spectrophotometer (UV-245 Shimadzu, Japan).
Ovarian Reduced Glutathione (GSH)
Briefly 200 μl of ovarian homogenate (20 mg/ml) was mixed with 100 µl of sulfosalicylic acid and the mixture was centrifuged for 10 min at 3000 rpm. Then in 200 μl of supernatant, 1.8 ml of DTNB was added and shaken well.24 The reading was taken at 412-420 nm.
Ovarian Superoxide Dismutase (SOD)
Superoxide dismutase was measured by the method of Marklund and Marklund. The SOD activity of the supernatant was estimated by measuring the percentage of inhibition of the pyragallolauto oxidation by SOD.25 The buffer contains 50 mM Tris HCl, 10 mM hydrochloric acid (HCl) in the presence of 1 mM EDTA. Then the buffer mixture (2 ml), 100 μl of 2 mM pyrogallol and 10 μl of ovarian homogenate were poured in a spectrophotometric cuvette and the reading was measured in the spectrophotometer (UV-245 Shimadzu, Japan) at 420 nm for 3 min.
Ovarian Catalase (CAT)
In a spectrophotometric cuvette, 1 ml of 30 mM H2O2, 1.9 ml of 50 mM phosphate buffer and 0.1 ml of ovarian homogenate (in 0.05M Tris HCl) were taken.26 After mixing, readings were noted at 240 nm at 30 sec interval.
Ovarian Glutathione-S-Transferase (GST)
In a spectrophotometric cuvette, 0.1 ml of ovarian homogenate, 0.2 ml 100 mM PBS, 0.05 ml of 1 mM GSH and 0.02 ml of 60 mM CDNB was taken in a cuvette and reading was noted at 340 nm. The values were expressed in µmol CDNB conjugate formed/min/ mg protein.27
Ovarian Glutathione Peroxidase (GPx)
At first 0.2 ml of 0.4 M phosphate buffer (pH-7), 0.1 ml of 10 mM sodium azide, 0.2 ml of ovarian homogenate (20 mg/ml) in phosphate buffer (pH-7), 0.2 ml of 4 mM reduced glutathione and 0.1 ml of 2.5 mM H2O2 were taken, mixed and incubated for 10 min at 37 ºC.28 Then 0.4 ml of 10% TCA was added to stop the reaction and centrifuged at 3200 rpm for 20 min. Then 1 ml of 5, 5‘-dithiobisnitrobenzoic acid (DTNB) and 3 ml of disodium hydrogen phosphate (Na2HPO4) were added to 0.5 ml of supernatant. The absorbance was measured at 420 nm.
Ovarian Glutathione Reductase (GR)
Briefly 2 ml of oxidized glutathione (GSSG), 20 µl of 12 mM NADPH.Na4 and 2.68 ml of phosphate buffer (PBS) were added with 100 µl of ovarian homogenate and the reading was taken at 340 nm.29
Statistical Analysis
The results were expressed as the Mean±SEM. Statistical analyses of the collected data were done by one-way analysis of variance (ANOVA) followed by multiple comparison t-tests. Difference was considered significant when p<0.05.
RESULTS
Effects on the Age of Vaginal Opening and Appearance of First Estrous
Table 1 shows the effect of cypermethrin on vaginal opening and appearance of first estrous in female prepubertal rat. Compared with the control group, cypermethrin (low and high dose) exposure delayed the age of vaginal opening and appearance of first estrous significantly (p<0.01) in a dose-dependent manner. On the other hand, pretreatment of zinc showed comparatively earlier age of vaginal opening and appearance of first estrous.
| Table 1: The effect of zinc on average duration of first estrous cycle phases and vaginal opening in cypermethrin induced female prepubertalrats |
|
First estrous (age in days) |
Vaginal opening (age in days) |
| Group-I (5 ml /kg body wt.) |
65.06±0.42 |
49.42±0.561 |
| Group-II [Zinc (227 mg/l in drinking water control] |
64.28±0.283 |
47.42±0.814 |
| Group-III [Cypermethrin-treated (34.33mg/ kg body wt., Low dose) group] |
73.46±0373 a* |
53.3±0.478 a** |
| Group-IV [Zinc+cypermethrin-treated (34.33mg/ kg body wt., Low dose) group] |
68.51±0.402 b* |
50.43±0.4 a* |
| Group-V [Cypermethrin-treated (51.5mg/ kg body wt., High dose) group] |
80.32±0.645 a*** |
66.71±0.586 a*** |
| Group-VI [Zinc+Cypermethrin-treated (51.5mg/kg body wt., High dose) group] |
75.15±0.485 a* c** |
54.4±0.822 a**c** |
| Results are expressed as Mean±SEM. Analysis is done by ANOVA followed by multiple comparison two-tail t-tests. Superscript a Group I versus all other groups; superscript b Group III versus Group IV and superscript c Group V versus Group VI (*indicates p<0.05, ** indicates p<0.01 and *** represents p<0.001). |
Reproductive Organ Weights
In cypermethrin treated high dose group ovarian index, uterine index, adrenal index were decreased significantly compared to the control group. Pre-treatment of zinc improved these indices in cypermethrin-induced rat (Table 2).
| Table 2: Illustrates the effect of zinc on ovarian, uterine and adrenal indicesin cypermethrin induced female prepubertal rats. |
|
Ovarian Index |
Uterine Index |
Adrenal Index |
| Group-I (5 ml /kg body wt) |
32.33±0.071 |
79.66±0.05 |
16.65±0.059 |
| Group-II [Zinc (227 mg/l in drinking water control] |
30.058±0.052 |
77.78±0.035 |
15.25±0.044 |
| Group-III [Cypermethrin-treated (34.33 mg/ kg body wt.,Low dose) group] |
24.138±0.041 a** |
59.63±0.045 a** |
15.01±0.049 |
| Group-IV [Zinc+Cypermethrin-treated (34.33 mg/ kg body wt., Low dose) group] |
28.1±0.07 b* |
66.64±0.043 a* b* |
15.11±0.043 |
| Group-V [Cypermethrin-treated (51.5 mg/ kg body wt.,High dose) group] |
18.641±0.06 a*** |
49.14±0.039 a*** |
12.34±0.04 a* |
| Group-VI [Zinc+Cypermethrin-treated (51.5 mg/ kg body wt., High dose) group] |
22.25±0.06 a** c* |
53.15±0.039 a**c* |
13.25±0.045 a* |
| Results are expressed as Mean±SEM. Analysis is done by ANOVA followed by multiple comparison two-tail t-tests. Superscript a Group I versus all other groups; superscript b Group III versus Group IV and superscript c Group V versus Group VI (*indicates p<0.05, ** indicates p<0.01 and *** represents p<0.001). |
Ovarian and Adrenal Cholesterol Content
Cypermethrin caused an accumulation of cholesterol (p<0.01) in the ovary (Figure 1) of female prepubertal rats, whereas the adrenal cholesterol (Figure 2) in cypermethrin exposed groups were decreased (p<0.01). Zinc ameliorated cypermethrin-induced cholesterol accumulation in rat.
Figure 1: The effect of zinc on ovarian cholesterol content in cypermethrin induced female prepubertal rats.
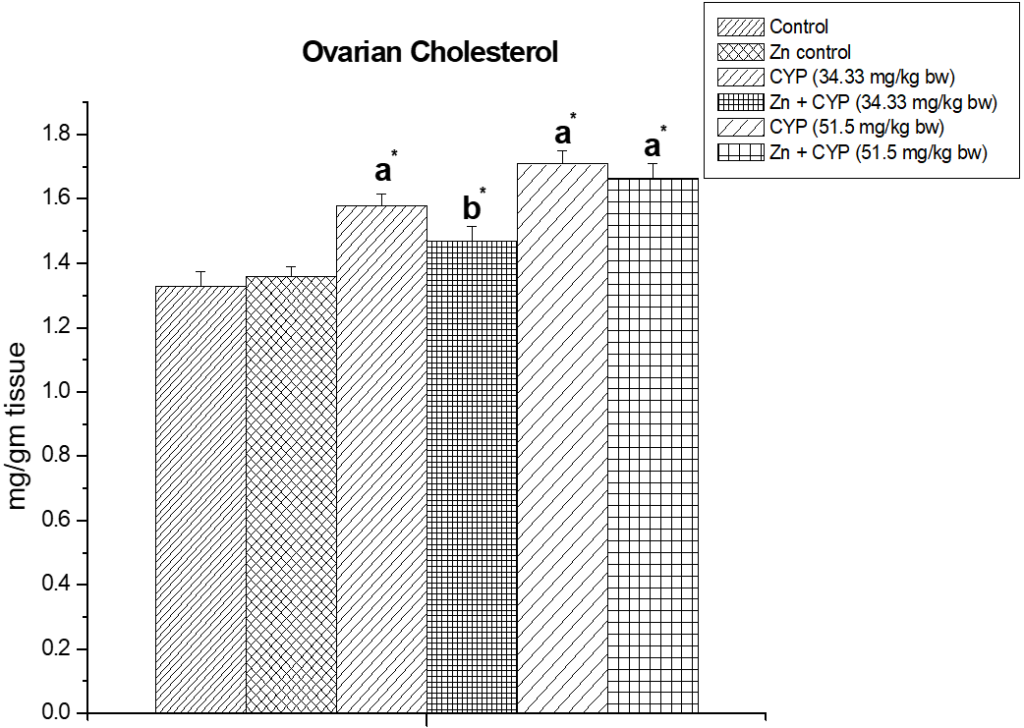
Results are expressed as Mean±SEM. Analysis is done by ANOVA followed by multiple comparison two-tail t-tests. Superscript a Group I versus all other groups; superscript b Group III versus Group IV (*indicates p<0.05).
Figure 2: The effect of zinc on adrenal cholesterol content in cypermethrin induced female prepubertal rats.
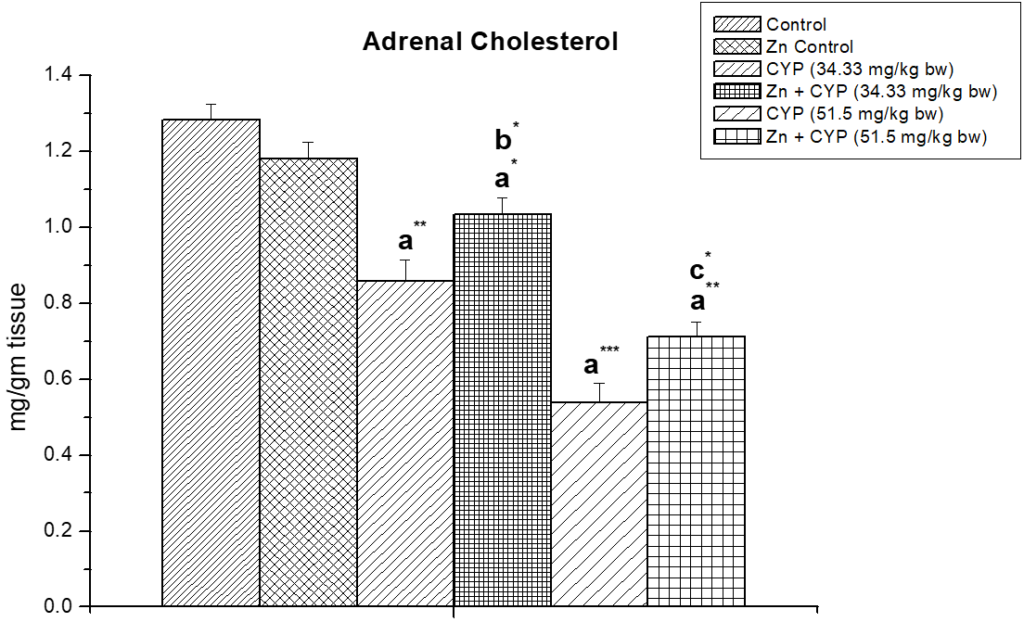
Results are expressed as Mean±SEM. Analysis is done by ANOVA followed by multiple comparison two-tail t-tests. Superscript a Group I versus all other groups; superscript b Group III versus Group IV and superscript c Group V versus Group VI (*indicates p<0.05, ** indicates p<0.01 and *** represents p<0.001).
Ovarian and Adrenal Ascorbic Acid Content
Cypermethrin (high dose) caused an accumulation of ascorbic acid content (p<0.01) in the ovary (Figure 3) of female prepubertal albino rats, whereas the ascorbic acid of adrenal gland (Figure 4) were decreased (p<0.01). Pre-treatment of zinc restored the status in cypermethrin-induced rat.
Figure 3: Illustrates the effect of zinc on ovarian ascorbic acid content in cypermethrin-exposed female prepubertal rats.
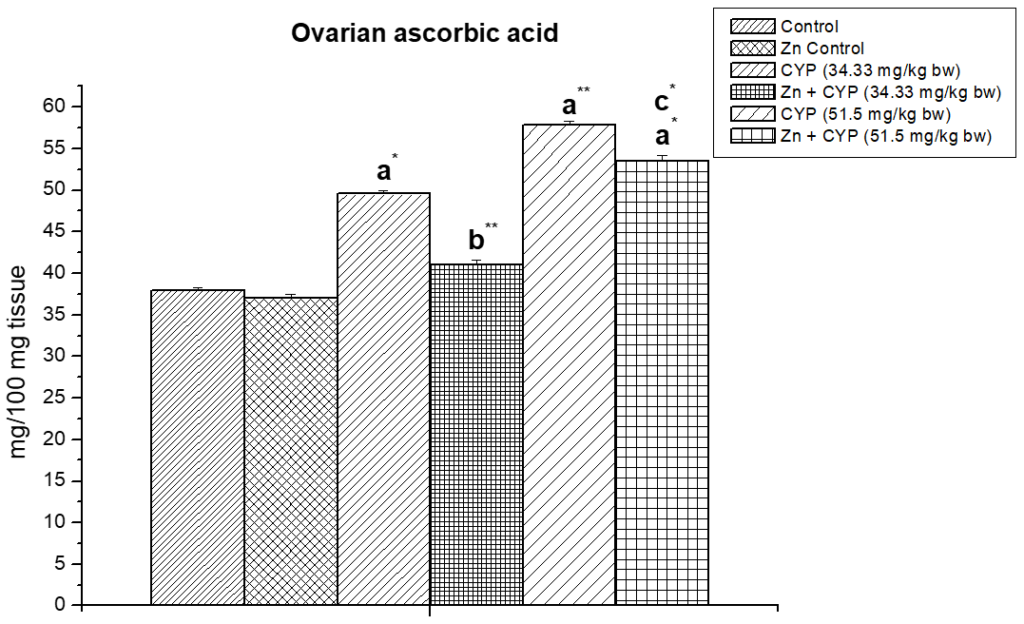
Results are expressed as Mean±SEM. Analysis is done by ANOVA followed by multiple comparison two-tail t-tests. Superscript a Group I versus all other groups; superscript b Group III versus Group IV and superscript c Group V versus Group VI (*indicates p<0.05, ** indicates p<0.01).
Figure 4: Effect of zinc on adrenal ascorbic acid content in cypermethrin induced female prepubertal rats.
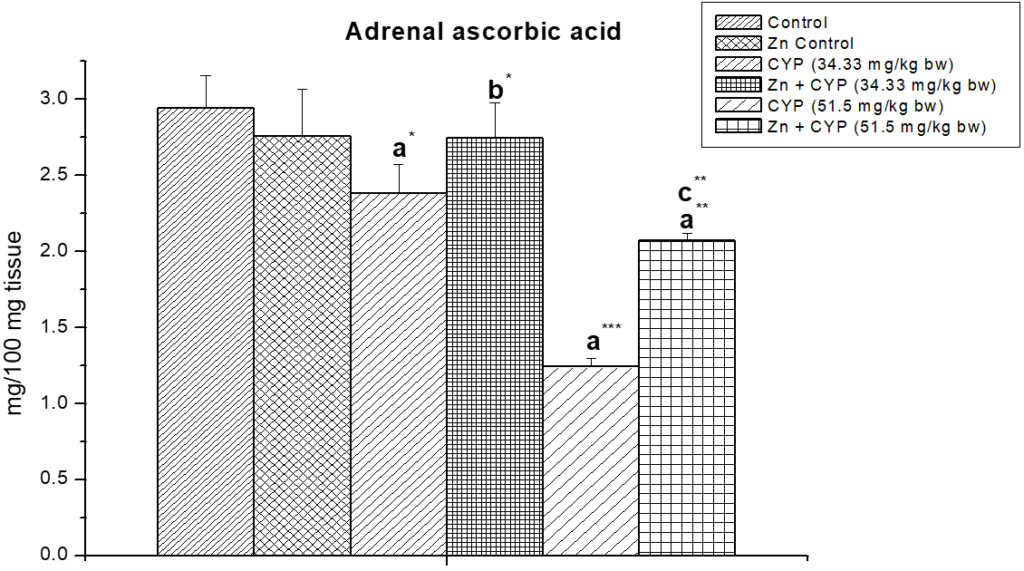
Results are expressed as Mean±SEM. Analysis is done by ANOVA followed by multiple comparison two-tail t-tests. Superscript a Group I versus all other groups; superscript b Group III versus Group IV and superscript c Group V versus Group VI (*indicates p<0.05, ** indicates p<0.01 and *** represents p<0.001).
Ovarian and Adrenal ∆5,3β-HSD and 17β-HSD Activity
The treatment of prepubertal female albino rats with cypermethrin (high dose) reduced the activities of ∆5,3β-HSD and 17 β-HSD (Figures 5, 6) enzymes in ovary and increased the activities of these enzymes (Figures 7 and 8) in the adrenal gland. Pre-treatment of zinc restored the activities of these enzymes in cypermethrin-induced rats.
Figure 5: Illustrates the effect of zinc on ovarian ∆5,3β-HSD in cypermethrin induced female prepubertal rats.
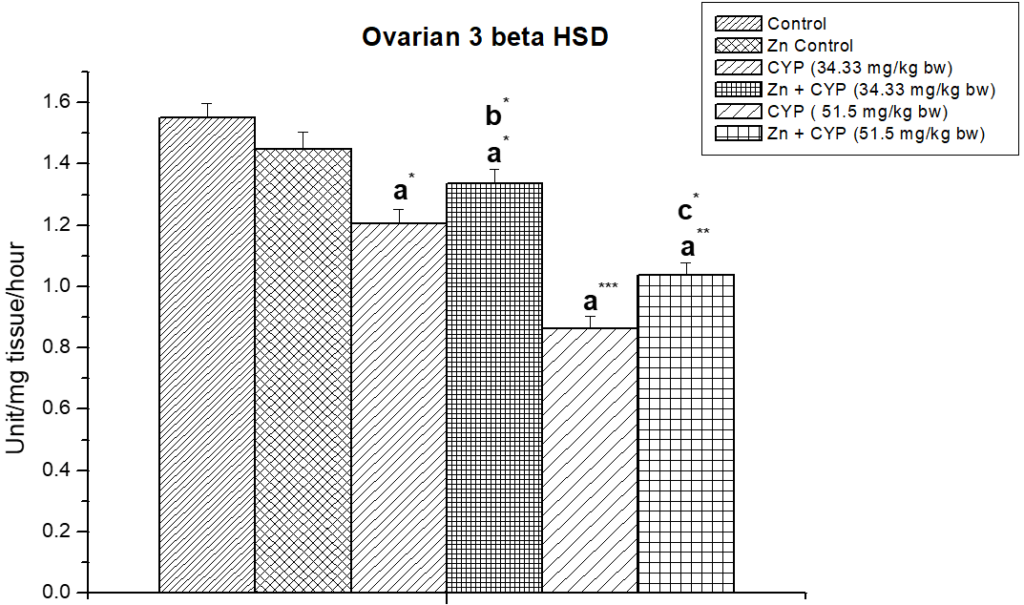
Results are expressed as Mean±SEM. Analysis is done by ANOVA followed by multiple comparison two-tail t-tests. Superscript a Group I versus all other groups; superscript b Group III versus Group IV and superscript c Group V versus Group VI (*indicates p<0.05, ** indicates p<0.01 and *** represents p<0.001).
Figure 6: Effect of zinc on ovarian 17β-HSD in cypermethrin-exposed female prepubertal rats.
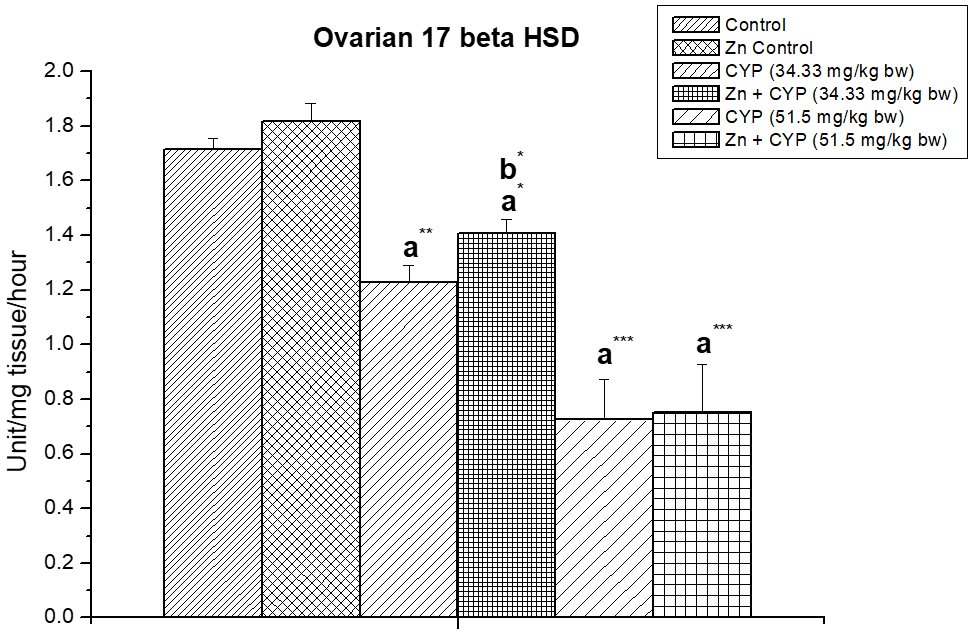
Results are expressed as Mean±SEM. Analysis is done by ANOVA followed by multiple comparison two-tail t-tests. Superscript a Group I versus all other groups; superscript b Group III versus Group IV (*indicates p<0.05, ** indicates p<0.01 and *** represents p<0.001).
Figure 7: The effect of zinc on adrenal ∆5,3β-HSD in cypermethrin induced female prepubertal rats.
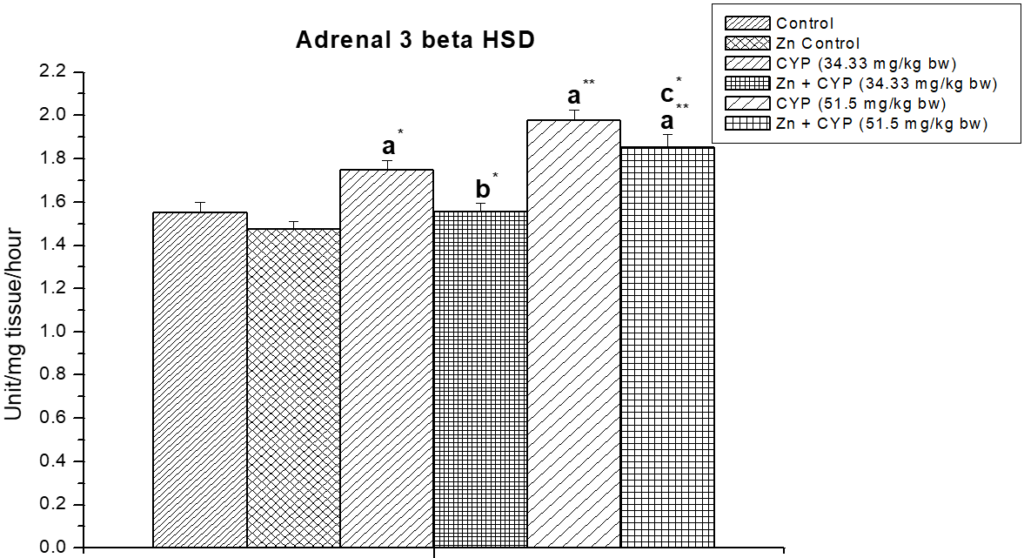
Results are expressed as Mean±SEM. Analysis is done by ANOVA followed by multiple comparison two-tail t-tests. Superscript a Group I versus all other groups; superscript b Group III versus Group IV and superscript c Group V versus Group VI (*indicates p<0.05, ** indicates p<0.01).
Figure 8: Illustrates the effect of zinc on adrenal 17β-HSD in cypermethrin-exposed female prepubertal rats.
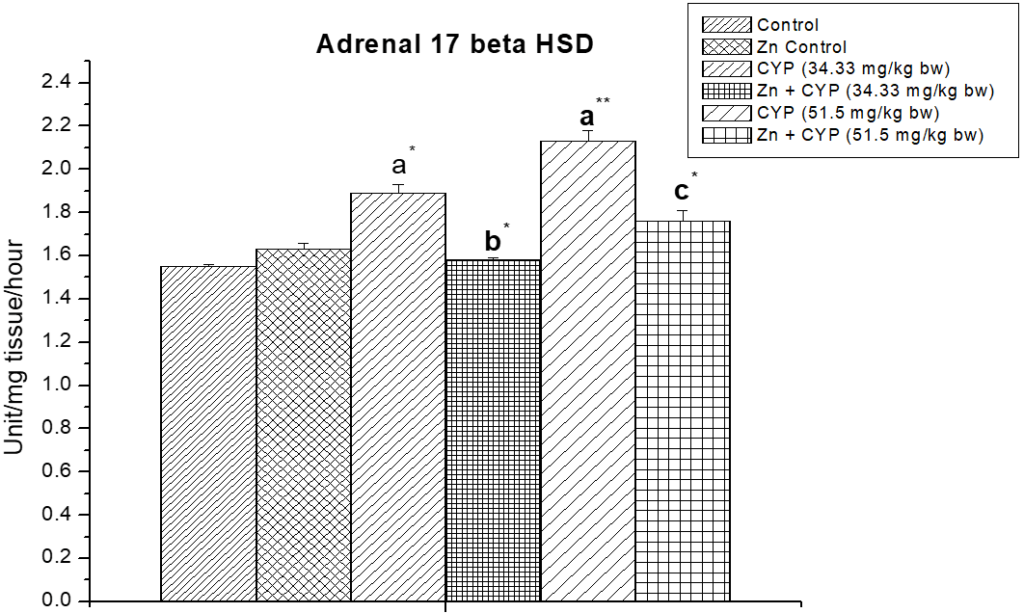
Results are expressed as Mean±SEM. Analysis is done by ANOVA followed by multiple comparison two-tail t-tests. Superscript a Group I versus all other groups; superscript b Group III versus Group IV and superscript c Group V versus Group VI (*indicates p<0.05, ** indicates p<0.01).
Effect on LH, FSH and Estrogen
The levels of luteinizing hormone (LH), follicle stimulating hormone (FSH) and estradiol (E2) were reduced significantly in rats of cypermethrin (high dose) treated group (Table 3). Pre-treatment of zinc restored these hormones towards normal status in cypermethrin-induced rat.
| Table 3: The effect of zinc on reproductive hormone levels in cypermethrin induced female prepubertalrats |
|
LH (mIU/ml) |
FSH (mIU/ml) |
Estrogen (pg/ml) |
| Group-I (5 ml /kg body wt) |
0.556±0.006 |
0.625±0.045 |
37.5±0.763 |
| Group-II Zinc (227 mg/l in drinking water control) |
0.561±0.006 |
0.7±0.005 |
37.16±0.703 |
| Group-III Cypermethrin-treated (34.33mg/ kg body wt.,Low dose) group |
0.44±0.005 a** |
0.446±0.006a** |
29.5±0.763a** |
| Group-IV Zinc + Cypermethrin-treated (34.33mg/ kg body wt., Low dose) group |
0.513±0.006a*b* |
0.608±0.006b** |
34.33±0.881a*b* |
| Group-V Cypermethrin-treated (51.5mg/ kg body wt.,High dose) group |
0.235±0.007a*** |
0.286±0.006a*** |
18.66±0.666a*** |
| Group-VI Zinc + Cypermethrin-treated (51.5mg/ kg body wt., High dose) group |
0.395±0.007a**c** |
0.415±0.007a**c*** |
27±0.577a**c** |
| Results are expressed as Mean±SEM. Analysis is done by ANOVA followed by multiple comparison two-tail t-tests. Superscript a Group I versus all other groups; superscript b Group III versus Group IV and superscript c Group V versus Group VI (*indicates p<0.05, ** indicates p<0.01 and *** represents p<0.001). |
Effects on Oxidative Stress
The effect of zinc on ovarian malon-di-aldehyde (MDA) in cypermethrin exposed female albino prepubertal rat is shown in Figure 9. MDA content increased significantly (p<0.01) in cypermethrin treated high dose group compared to the control group whereas pre-treatment of zinc decreased the cypermethrin toxicity and normalized the oxidative status of the ovary.
Figure 9: The effect of zinc on ovarian malon-di-aldehyde (MDA) level in cypermethrin induced female prepubertal rats.
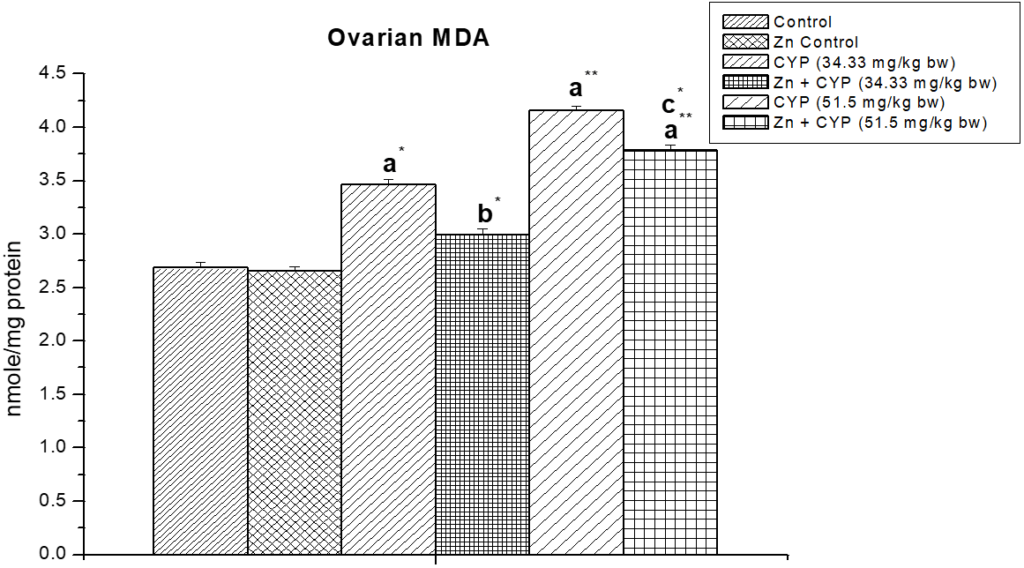
Results are expressed as Mean±SEM. Analysis is done by ANOVA followed by multiple comparison two-tail t-tests. Superscript a Group I versus all other groups; superscript b Group III versus Group IV and superscript c Group V versus Group VI (*indicates p<0.05, ** indicates p<0.01).
Figure 10 shows the ovarian GSH in cypermethrin exposed female albino prepubertal rat. From this figure it is observed that GSH were decreased significantly (p<0.05) in cypermethrin treated groups compared to control.
Figure 10: Effect of zinc on ovarian reduced glutathione (GSH) content in cypermethrin-exposed female prepubertal rats.
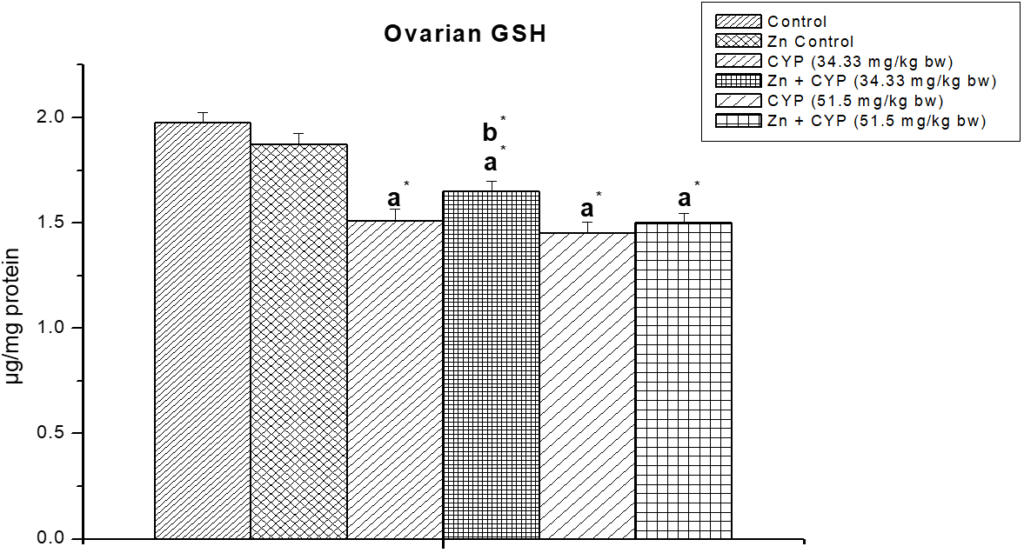
Results are expressed as Mean±SEM. Analysis is done by ANOVA followed by multiple comparison two-tail t-tests. Superscript a Group I versus all other groups; superscript b Group III versus Group IV (*indicates p<0.05).
As presented in Figure 11, the activity of SOD in the cypermethrin treated low and high dose were significantly decreased (p<0.01) and (p<0.001) compared to the control group. The activity of CAT (Figure 12) in the cypermethrin exposed low and high dose groups were significantly (p<0.001) decreased compared to the control group. However, pre-treatment with zinc with cypermethrin resulted in a significant increase in the activity of SOD, CAT. There was no significant changes found in GST level in cypermethrin treated low dose group but slight alteration has been found in cypermethrin treated high dose group (Figure 13).
Figure 11: The effect of zinc on ovarian superoxide dismutase (SOD) activity in cypermethrin induced female prepubertal rats.
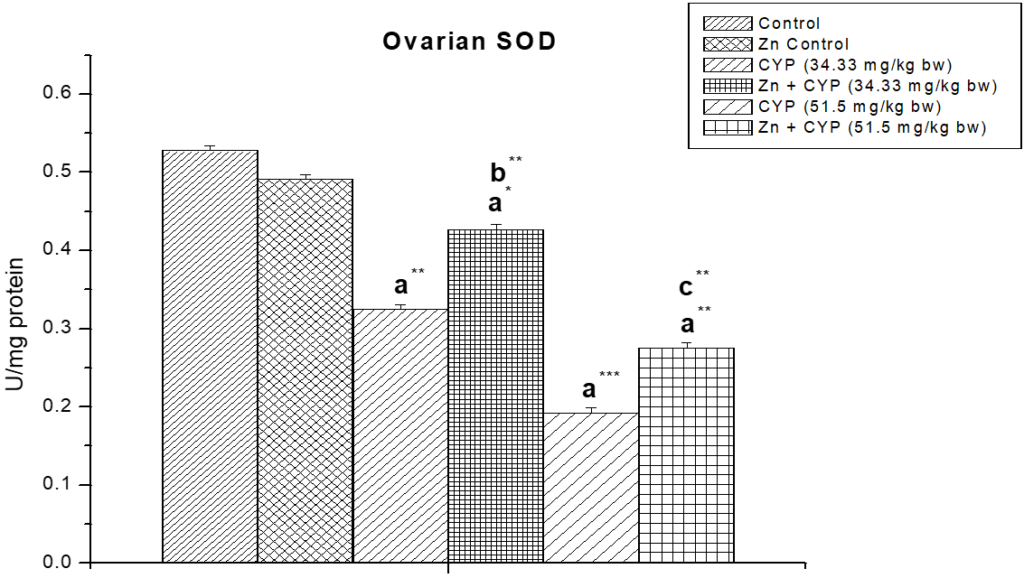
Results are expressed as Mean±SEM. Analysis is done by ANOVA followed by multiple comparison two-tail t-tests. Superscript a Group I versus all other groups; superscript b Group III versus Group IV and superscript c Group V versus Group VI (*indicates p<0.05, ** indicates p<0.01 and *** represents p<0.001).
Figure 12: Effect of zinc on ovarian catalase (CAT) activity in cypermethrin-exposed female prepubertal rats.
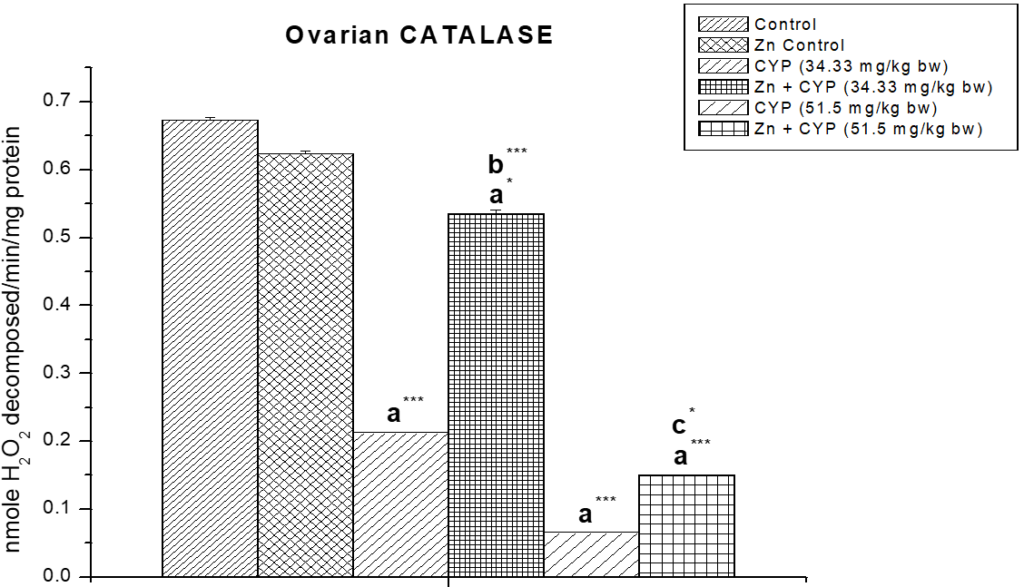
Results are expressed as Mean±SEM. Analysis is done by ANOVA followed by multiple comparison two-tail t-tests. Superscript a Group I versus all other groups; superscript b Group III versus Group IV and superscript c Group V versus Group VI (*indicates p<0.05 and *** represents p<0.001).
Figure 13: The role of zinc on ovarian glutathione-s-transferase (GST) activity in cypermethrin induced female prepubertal rats.
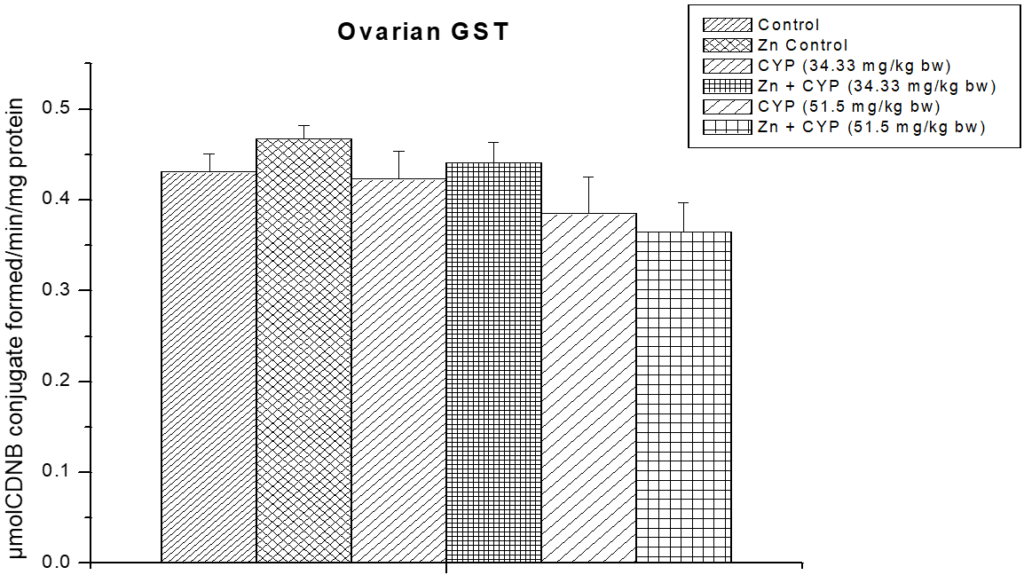
Results are expressed as Mean±SEM. Analysis is done by ANOVA followed by multiple comparison two-tail t-tests.
The activity of glutathione peroxides and glutathione reeducates (Figures 14 and 15) in the cypermethrin treated group was decreased in ovary whereas zinc resulted in a significant increase in the activity of these two enzymes.
Figure 14: The effect of zinc on ovarian glutathione peroxidase (GPx) activity in cypermethrin-exposed female prepubertal rats.
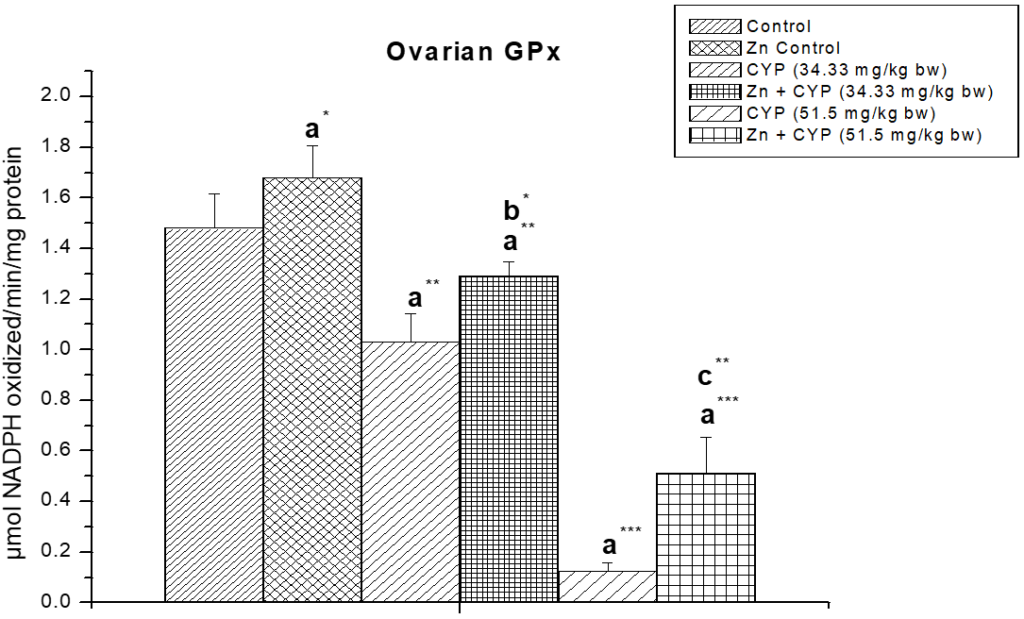
Results are expressed as Mean±SEM. Analysis is done by ANOVA followed by multiple comparison two-tail t-tests. Superscript a Group I versus all other groups; superscript b Group III versus Group IV and superscript c Group V versus Group VI (*indicates p<0.05, **indicates p<0.01 and ***represents p<0.001).
Figure 15: Effect of zinc on ovarian glutathione reductase (GR) activity in cypermethrin induced female prepubertal rats.
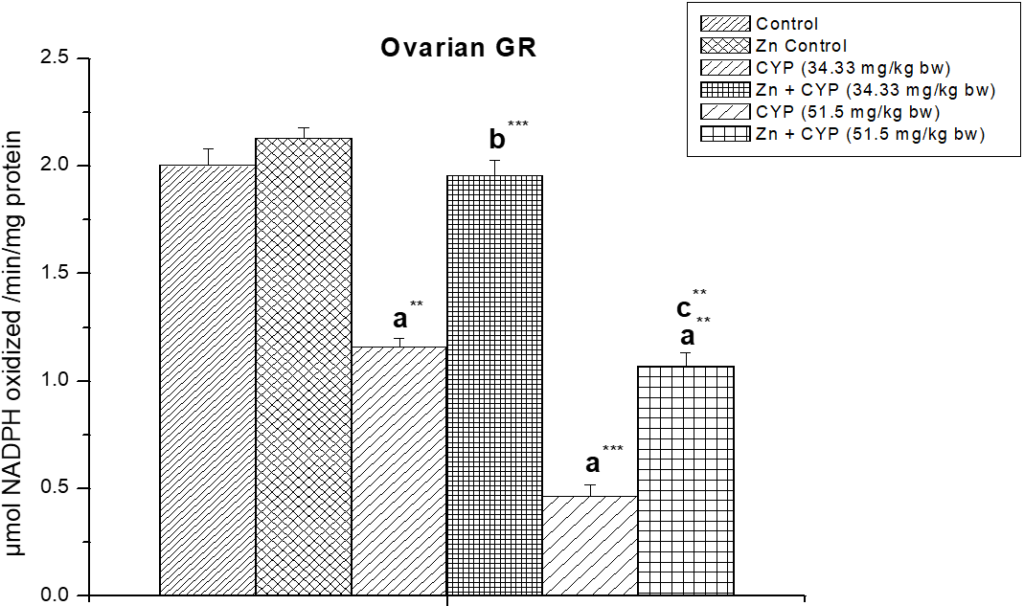
Results are expressed as Mean±SEM. Analysis is done by ANOVA followed by multiple comparison two-tail t-tests. Superscript a Group I versus all other groups; superscript b Group III versus Group IV and superscript c Group V versus Group VI (** indicates p<0.01 and *** represents p<0.001).
DISCUSSION
In this study decrease in ovarian index in case of cypermethrin treated rats compared to control may be due to decreased number of ovarian germ cells. Rat is a spontaneously ovulating species with estrous cycles and exhibits regular endocrine events. In this study, estrous cycle have been examined in the cypermethrin exposed female prepubertal rats as an indicator about the toxic effects of cypermethrin on the female reproductive function involving the component roles of hypothalamus, pituitary, ovary and uterus. The reduction in ovarian and uterine indices that may be due to reduced anabolic role of estradiol on the weight of the ovary and uterus.30 This decrease may also be due to other toxic effects of cypermethrin another systems of the animal (Table 2). In case of female prepubertal rats, exposure to cypermethrin remarkably delayed the onset of puberty as evidenced by the age of vaginal opening and appearance of first estrous (Table 1). The delay in the age of vaginal opening and appearance of first estrous may be related with the decline in the ovarian steroid genesis process.30
In the present study, the significant rise in ovarian cholesterol content of cypermethrin treated rats advocates the less utilization of cholesterol towards the biosynthesis of ovarian steroid hormones. Thus it outcomes the malfunctioning of ovarian steroidogenic activity of the cypermethrin treated rats. Ascorbic acid accumulates in the ovaries of treated rats which gives another support to the inhibition of steroidogenic activity.30 Pre-treatment of zinc decreased the cypermethrin toxicity and normalized the steroidogenic status of the ovary.
The low steroidogenic activity was also confirmed by the decreased activity of steroidogenic enzymes (∆5,3β-HSD, 17β-HSD). A reduced level of adrenal cholesterol and an increased activity of steroidogenic enzymes (∆5,3β-HSD, 17β-HSD) in adrenal cortex indicate the improvement of steroidogenes is by the adrenal gland.
Our studies stated that when cypermethrin was administered, serum. LH, FSH, and estrogen level significantly diminished. Zinc pre-treatment was able to restore the hormonal levels towards normal. From the results it may be considered that cypermethrin affected the set-point that regulate the secretion and function of some reproductive hormones secreted from the anterior pituitary and ovary that control the estrous cycle function in female rat.31 The significant reduction in the level of LH, FSH and estradiol (Table 3) in cypermethrin treated rats indicates that cypermethrin may inhibit the function of ovary and uterus. This is also supported by the results from estrous cycle study. Most probably this is accomplished by inhibition of the secretion of LH and FSH from anterior pituitary and estradiol from ovary. The depression of the secretion of LH and FSH might be due to direct action of cypermethrin on anterior pituitary gonadotrophs, responsible for the secretion of LH and FSH; or hypothalamic neurons, responsible for the secretion of gonadotropin-releasing hormone (GnRH) that exercises tropic action on anterior pituitary gonadotrophs.32
Diminution in GSH levels in ovary after cypermethrin treatment may be the indication of oxidative stress, whereas GSH is utilized for the detoxification of reactive toxic substances. As one of the most essential biological molecules, GSH play a key role in the detoxification of the reactive toxic metabolites. Normal cellular function is executed through a balance between ROS production and antioxidant defense mechanisms existing in the cell.33 There was no significant changes found in GST level in cypermethrin treated low dose group but slight change has been found in cypermethrin treated high dose group. From the results, the activities of ovarian SOD, CAT, glutathione peroxides and glutathione reductase of cypermethrin treated rats were significantly decreased. These results suggested that cypermethrin has the capability to persuade free radicals and oxidative insult as demonstrated by alterations in various antioxidant enzymes in ovary of prepubertal rats.
Zinc pre-treatment restored all these ovarian parameters towards normal level to a better extent. By scavenging or quenching free radicals, hydrogen peroxide or hypochlorous acid directly, or by binding free metal ion species like Fe2+ or Cu2+ by its sulfonic acid group, zinc reduced lipid peroxidation. By diminishing oxidative stress, it safeguards the tissue damage, as well as the ovarian toxicity.34,35
Thus, from the above mentioned results we may conclude that cypermethrin suppresses the female prepubertal reproductive function in rat probably by impairing the estrous cycle; inhibiting the secretion of female reproductive hormones and promoting the oxidative stress of the tissues of ovary. From the above findings, it is evident that cypermethrin cause prominent inhibition in ovarian steroidogenesis in a dose-dependent manner in prepubertal female Wistar rats and zinc has more potent ameliorative role on cypermethrin induced reproductive toxicity.
ACKNOWLEDGEMENT
The authors are grateful to the authority of Vidyasagar University, Midnapore, India for providing all the facilities to execute this study.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONSENT
The study was approved by the Institutional Animal Ethical Committee (IAEC), registered under Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment, Forests & Climate Change, Govt. of India and performed in compliance with the relevant laws and guidelines of the CPCSEA.




















