BACKGROUND
Hyponatremia has a large differential diagnosis and is a critical issue in cancer patients. The Syndrome of Inappropriate Secretion of Antidiuretic Hormone (SIADH) is a well-known cause of hyponatremia, while physicians generally overlook Renal Salt Wasting Syndrome (RSWS). This reflects the difficulty of differentiating between SIADH and RSWS based on limited clinical or laboratory findings. This case illustrates a useful way of discriminating between these two disorders and also shows that carboplatin is an extremely rare cause of RSWS.
CASE PRESENTATION
A 40-year-old woman was admitted to our hospital for chemotherapy. Two years ago she was diagnosed with small cell lung cancer (cT4N3M1a, stage IV, extensive stage) and was treated with 5 cycles of cisplatin (CDDP) plus etoposide (VP-16), followed by two cycles of carboplatin (CBDCA) plus VP-16. The latest chemotherapy was performed three weeks prior to current admission, and the lung cancer had been assessed as stable disease based on the evaluation criteria of solid tumours (RECIST) guidelines.1 However, she had multiple episodes of hyponatremia (minimum serum sodium: 116 mEq/L) whenever she received chemotherapy for the preceding year (total of five episodes). She was presumptively diagnosed with SIADH, and she improved with restriction of water intake in addition to supplementation of sodium levels at each time. At her current admission, the vital signs were normal, and physical examination showed a moderate pleural effusion in her right hemithorax. Laboratory examinations were normal except for moderate anemia (8.2 g/dL) and leukocytopenia (1.9×103/μL). Her serum sodium was also normal (138 mEq/L) with oral intake of sodium (6 g/day). At this point, she did not satisfy the criteria for SIADH. Therefore, to investigate the differential diagnosis of the hyponatremia expected in due course with chemotherapy, an intensive survey for hyponatremia was performed at the day of admission using a 24-hour urine collection. From days 3 to 5, she was treated with intravenous CBDCA plus VP-16. On day 6, she had nausea and arthralgia and the hyponatremia (130 mEq/L) emerged together with hypouricemia (2.9 mg/dL) and increased fractional excretion of urate (FEurate) (13%>10%) (Figure 1). However, on the same day, thyroid function (free T4: 1.23 ng/dL, free T3: 2.99 pg/mL, and thyroid stimulating hormone: 31.8pg/mL), serum cortisol (6.8 mg/dL), aldosterone (48.5 pg/mL), renin activity (0.4 ng/mL/hr), and brain natriuretic peptide (74.8 pg/mL) were all within normal limits. The 24-hour urine collection showed that urine osmolality (429 mOsm/kg) was higher than serum osmolality (263 mOsm/kg), and urine sodium (97 mmol/L) and chloride (87 mmol/L) levels were also high. No elevation of tubular enzymes were noted.
Figure 1: The clinical course of the present case.
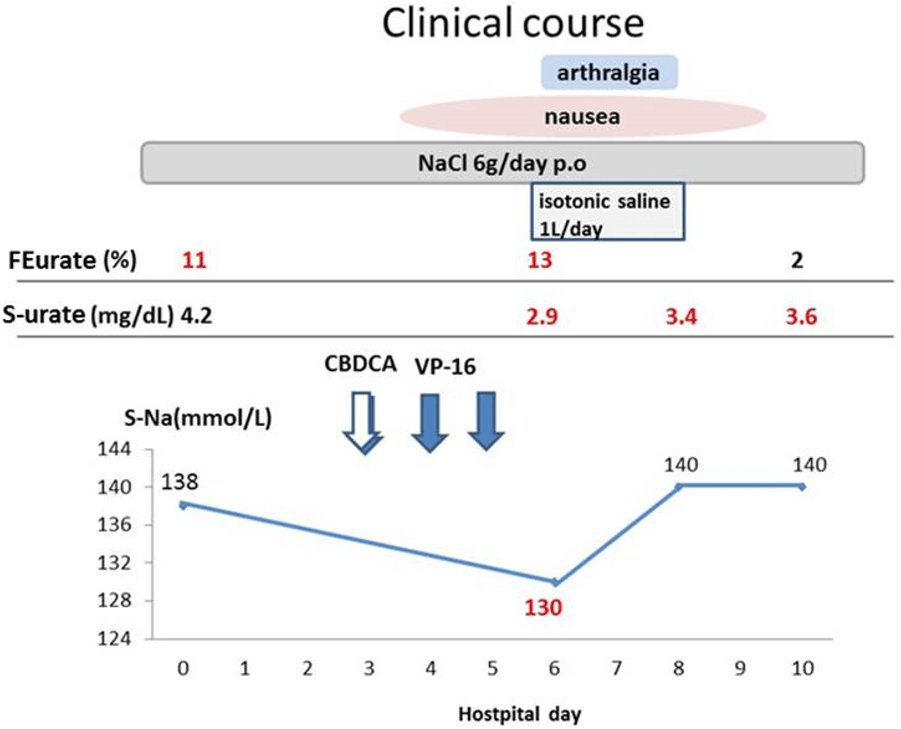
FENa: Fractional excretion of sodium, S-urate: serum urate.
During the clinical course, presence of decreased Extra Cellular Volume (ECV) was assessed by: 1) decreased skin turgor; 2) dry axilla; 3) delayed capillary refill time (>2 sec); 4) dry mouth; 5) postural hypotension;and 6) jugular venous pressure. None of these were recognized from day 0 to day 10, so there was no severe depletion of ECV was noted throughout this phase. FENa (fractional excretion of sodium)>1% was seen from admission to the day 10 (day 0, 6, 8, and 10).
Based on the findings of hypouricemia (<4 mg/dL), increased FEurate (>10%), and high urine sodium (>40 mmol/L) in addition to hyponatremia, RSWS was suspected due to CBDCA or SIADH. Table12,3,4,5 compares expected laboratory values for RSW and SIADH in hyponatremic patients, with the red letters consistent with the present case on day 6. After initiation of volume repletion therapy with isotonic saline (1 L/day for two days; day 6 and 7), the hyponatremia improved rapidly to 140 mEq/L over two days. This indicated that the hyponatremia was caused by RSWS, but not by SIADH.
| Table 1: Differentiation of SIADH from RSWS. |
| |
RSWS
|
SIADH
|
| Clinical Features |
| ECV |
↓
|
N-↑
|
| Postural hypotension |
+
|
–
|
| Laboratory findings |
|
|
| Plasma renin
(normal, 2.8-26.7 mU/L) |
N-↑
|
N-↓
|
| Plasma aldosterone
(normal, 10 to 160 ng/L) |
N-↑
|
±↓
|
| Serum urate |
↓↓
|
N-↓
|
| FE urate (normal 5-10%) |
↑↑
|
N-↑
|
| FE phosphate (normal <20%) |
N-↑
|
N
|
| UNa |
N-↑
|
N-↑
|
| Serum Na after treatment with isotonic saline |
↑
|
→ - ↓
|
| ADH |
N-↑
|
↑
|
| ANP/BNP |
N-↓
|
N-↑
|
| Treatment |
Salt loading/volume replacement |
Free water restriction/hypertonic saline infusion |
| ADH: Antidiuretic Hormone, ANP: Antinatriuretic Peptide, BNP: Brain Natriuretic Peptide, FE: Fractional Excretion, N: Normal range, RSWS: Renal Salt Wasting Syndrome, SIADH: Syndrome of Inappropriate Secretion of ADH, UNa: Urine sodium |
DISCUSSION
Small cell lung cancer is a disseminated disease in most patients and the primary therapeutic modality is systemic chemotherapy for extensive stage disease as in the present case. The standard regimens are platinum-based combinations (CDDP plus VP-16 or CBDCA plus VP-16) because of their activity and toxicity profile.6 Carboplatin (CBDCA) has less nephrotoxicity and ototoxicity than cisplatin, with greater or comparable antitumor activity.7 However, sporadic observations of renal function deterioration after multiple courses of CBDCA have been reported, with reductions in the glomerular filtration rate or effective renal plasma flow.8,9 During the management of cancer patients, hyponatremic status needs to be carefully considered because it can be caused by diverse disorders, as well as concomitant use of other drugs acting on the serum sodium level.10,11 Among these disorders, differentiating between SIADH and RSWS is a critical issue because of their opposite treatments (i.e. fluid restriction done for management of SIADH can worsen the hyponatremia in RSWS, whereas fluid replacement with isotonic saline done for management of RSWS can worsen the hyponatremia in SIADH). From this perspective, the hyponatremia of the present case showed immediate correction via intravenous infusion of isotonic saline (1 L/day), suggesting RSWS (Figure 1).
The elevated plasma renin, aldosterone, and ADH and low normal atrial natriuretic peptide levels are all physiologic consequences of RSWS.2,3,4,5 However, the extent of Extracellular Volume (ECV) depletion in RSWS seems to depend on the severity of renal dysfunction of sodium reabsorption. Furthermore, physical assessment of ECV is limited, which leads to a therapeutic dilemma: to restrict water for SIADH or to administer isotonic saline in RSW, especially in mild RSWS with normal to slightly depleted ECV, as in the present case.
Previous studies showed that no increase in tubular enzymes (β2-microglobulin or N-acetyl-β-glucosaminidase (NAG)) was found in patients receiving CBDCA up to 520 mg/m2,7,12 but data on patients who developed CBDCA nephrotoxicity is unavailable. Santana et al. reported that 40% of patients receiving high-dose CBDCA and VP-16 with autologous marrow transplantation with relapsed solid tumours had hyponatremia,13 but no reason was given.
To the best of our knowledge, only two cases of RSWS associated with CBDCA have been reported with14 or without15 elevation of tubular enzymes, as can be seen with tubular damage. The main mechanism of nephrotoxicity due to CDDP has been reported to be damage of the proximal tubules that process the Organic Cation Transporter (OCT2), which serves as a critical transporter for CDDP uptake in both animals and humans,16,17 resulting in renal wasting of sodium, urate, or phosphate. Meanwhile, the CBDCA has no interaction with human OCT2 and reduces their entry into renal tubular cells.16,17 Therefore, the precise mechanism of CBDCA nephrotoxicity is unknown, but another unknown transporter-mediated process or passive diffusion might be associated with renal dysfunction.
The present case had mild impaired renal dysfunction on admission (FEurate>10%), which would be derived from tubular damage due to either the antecedent 5 cycles of chemotherapy with CDDP plus VP-16 (5th cycle was completed 3 months earlier) or the latest chemotherapy (3 weeks earlier) with CBDCA plus VP-16. Most previous cases of RSWS due to CDDP occurred from a few days to 10 days after completion of the chemotherapy and renal function recovered within the next three weeks.18,19 Thus, in the present case, the CBDCA seemed to be attributed to renal damage, and the renal dysfunction recovered at day 10 along with normalized FEurate or serum sodium. Sleijfer et al9 reported that cumulative dose of CBDCA cause considerable loss of renal function with no increase in tubular enzymes or changes in the relative β2-microglobulin clearances, which might be have occurred in the present case.
This case reminds us of the importance of the concept of “RSWS”, which is difficult to diagnose but treatable.
CONFLICTS OF INTEREST
None






