BACKGROUND
Chronic Pancreatitis (CP) is often associated with pain due to pancreatic duct obstruction. In these patients, surgical drainage was more effective than endoscopic treatment, achieving a faster, effective, and sustained pain relief.1 However, when surgery is not suitable, different endoscopic procedures could be performed. Endoscopic drainage usually requires transpapillary access to the pancreatic duct during Endoscopic Retrograde Cholangio-Pancreatography (ERCP). The main limitation of endoscopic procedure is that the pancreatic duct could not be accessible at ERCP because of Roux-en-Y reconstruction after gastric-pancreatic surgery. Interventional Endoscopic Ultrasound (EUS) may allow a successful drainage of a dilated pancreatic duct, by using an endoscopic cysto-enterostomy followed by stent placement. We described the EUS-pancreaticogastrostomy performed in a patient who underwent a subtotal gastrectomy complaining with chronic pancreatitis.
CASE REPORT
A 60 year-old man with previous diagnosis of chronic pancreatitis presented with upper abdominal pain due to pancreatic duct dilation after sub-total gastrectomy with Roux-en-Y reconstruction for gastric cancer performed 15 year before. According to postsurgical anatomy, a first endoscopic attempt with colonscope (EC38-i10M, 160 cm, 3.8 mm, Pentax Medical) to the site of pancreatico-jejunal anastomosis failed because of afferent loop length. Therefore, we performed a transgastric EUS approach using a linear echo endoscope (EG-3870 UTK, 3.8 mm, Pentax Medical) showing a pancreatic duct ectasia of 9 mm and multiple stones into the lumen (Figure 1). After EUS-guided transgastric puncture of the pancreatic duct using a 19 G needle (EUS-19-T; Wilson Cook Medical Inc.), the guide wire was inserted into the pancreatic duct (pancreatic fluid was aspirated to confirm location) and a dilation was performed with a 10 Fr cystoenterostome (Cystatin-10 (CST-10); Wilson Cook Medical Inc.). At the end of the procedure, a metallic stent (Niti-S Biliary Covered Stent, Toewoog Medical, 12 mm diameter, 20 mm length) was placed using the fluoroscopy guide (Figure 2). The following pancreatography showed the correct position of the stent. The patient was discharged 2 days after the procedure, and the postoperative course was uneventful. He remained symptoms free for 24 months. Thereafter, an inflammatory stenosis of the main pancreatic duct was found. A pneumatic dilation of the stricture was performed, and a second stent (Wallflex, Boston Scientific, fully-covered, metal stent, 10 mm diameter, 40 mm length) was successfully placed.
Figure 1: Fluoroscopic view of EUS-guided transmural drainage
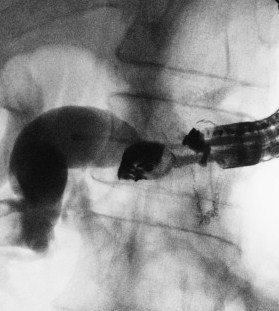
A. The echoendoscope is at the bottom of the gastric remnant and the contrast medium flows in the afferent loop
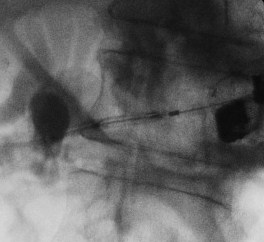
B. Performing dilation with cystoenterostome
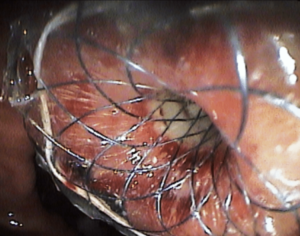
Figure 2: Endoscopic view of the fully covered stent placed in site
DISCUSSION
In the international literature, there are many articles about EUS-guided PG. The safety of the procedure is well-known in patients with chronic pancreatitis without previous surgery already more than 10 years ago.2 However, the use in surgical patients is newer and it has been described in the last years.3,4,5 The only paper with a “pure” small surgical casistic4 confirmed feasibility of this technique with a success rate of 67%.4 Other papers contain surgical patients3,5 in bigger series with higher success rates. However, the authors did not consider a specific success rate and morbidity for the operated class of patients. Another limitation is about the type of reconstruction: all the papers consider patients who have undergone Whipple intervention, but none of the authors specify the type of reconstruction. After the demolitive phase, in fact, two types of reconstructions are allowed: Billroth II and Roux-en-Y. This is a crucial point for subsequent endoscopic procedures: in case of Billroth II reconstruction, the endoscopist finds an afferent and an efferent loop at the bottom of the gastric remnant, and the afferent loop is generally shorter but it could be more angulated than in the other type of reconstruction; in case of Roux-en-Y reconstruction, instead, the endoscopist finds only one intestinal loop anastomized with the gastric remnant and the afferent loop is more distal and sometimes longer than in Billroth II, and so, more challenging to reach.
In patients with severe chronic pancreatitis who previously underwent upper gastrointestinal surgery, endoscopic treatment could be an alternative to a surgical re-intervention.2 Because of technical difficulties in performing the papillary approach in these patients, the use of EUS may easy allow a correct identification of the dilated pancreatic duct. We do not agree with the use of enteroscope because it does not allow the endoscopist to use large diameter stents. Technical success of EUS-guided pancreatic drainage was reported to range from 25% to 100%, with complications developing in 15% to 50% of patients.2,6,7 Most of technical failures were related to unsuccessful manipulation of the guidewire, whereas complications – mainly pancreatic leaks – depend on management of the transmural fistula.4 An initial insertion of a plastic stent in order to stabilize the created fistula is widely described in the literature.2-11 However, we found that a direct metallic stent positioning is possible, with less risk of pancreatic leak after the procedure. In addition, we believe that metal stents can drain longer and better than plastic stents, due to a greater diameter.6,10,11 Since pancreatic duct obstruction may occur in chronic pancreatitis, we would also suggest to leave the metallic stent in site.6 Such a stent may be easily identified when a re-stenting in needed. Indeed, we found that EUS guided puncturing of the main pancreatic duct from the gastric remnant is feasible, and it seems to be safe. Further studies are needed to confirm our encouraging data and we are organizing a monocentric case series. In conclusion, EUS-pancreaticogastrostomy is safe also in surgical patients whenever endoscopic reaching of the peri-ampullary area is dangerous and does not guarantee technical success.
CONFLICTS OF INTEREST
All the authors declare no conflicts of interest regarding the paper “Eus-Pancreaticogastrostomy in a Patient with Sub-total Gastrectomy and Roux-En-Y Reconstruction”.
CONSENT
The patient has provided written permission for publication of the case details.








