INTRODUCTION
Current data suggests that the amygdala is a central hub for emotional processing, decision making and fear. Very little research has been done that focuses on the pediatric and adolescent amygdala of psychiatric patients while in the hospital setting. We seek to analyze two specific groups of pediatric patients with resting state-functional magnetic resonance imaging (rs-fMRI). It is our hypothesis that patients who were hospitalized for a psychiatric cause would have increased perfusion as seen on functional magnetic resonance imaging (fMRI) when compared to a group of patients with intractable epilepsy. Patients with psychiatric disorders needing inpatient care usually complain of fear, anxiety and aggression.1 There are multiple studies reporting structural and biochemical amygdala changes in patients with schizophrenia,2 bipolar disorders,3 and mood dysregulation.4 A 2010 publication used emotional auditory stimuli to analyze brain activation in patients with schizophrenia. They found an increase of activity in the parahippocampal gyrus and the amygdala during the emotional session.5 Auditory and gustatory hallucinations have been also found to be associated with amygdala activation.6,7 It has been reported that patients with schizophrenia have augmented connectivity between the amygdala and the visual cortex.8
Despite these findings, there is not a clear explanation of the role played by the amygdala in psychosis. Even in the event of observable increased fMRI activation of the amygdala in psychiatric patient, it is difficult to know if the observation is the cause or the effect of the fear, anxiety or aggression. Patients with intractable epilepsy are subject to chronic very stressful situations that in some non-infrequent occasions may even threaten the patient’s life. In the present study, we want to compare in a pediatric population the frequency of activation of the amygdala in rs-fMRI studies between a group of patients received in our inpatient service of psychiatry and a group of patients with intractable epilepsy.
The amygdala is known to be involved in the fear response in a typical neurological circuit. Rs-fMRI is currently a recognized tool for non-invasive mapping of eloquent cortex. Its usage is mostly limited for pre-operative work-up in cases of tumors or epilepsy surgery. The standard fMRI requires the performance of certain tasks during the MRI acquisition. A variant of fMRI known as rs-fMRI may provide information of several functional areas including motor, sensory, visual and auditory without the involvement of any task. The technique may also provide insight in more complex networks that most likely relate to elaborated cognitive functions. These include executive functions, salience networks, basal ganglia, cerebellum and the default mode. The latter has called the attention of the scientific community as it decreases its activity when the subject is involved in a cognitive loaded task. One of the most popular techniques to extract these networks is called independent component analysis (ICA). ICA identifies groups of spatially correlated voxels that are oscillating in synchrony with other remote voxels groups. This phenomenon is known as spontaneous brain oscillations. The frequency of these oscillations is characteristically found between 0.01 and 0.1 Hz. Each extracted group sharing a single profile of oscillation is a functional network. Some of these networks are more stable than others (mostly those depicting unimodal brain functions) while others are less stable not appearing in all volunteers nor in all sessions of the same subject.
The amygdala network is an unstable network. There are no reports of formal assessment of the frequency of this activation. Our group has classified the networks obtained by rs-fMRI activation on 40 normal subjects and found that the amygdala network is only present in 10% of cases. It usually activates along with the ventral aspect of the anterior striatum9 and is usually symmetric.
METHODS
Psychiatric Group
For the sake of this study, a psychiatric case was defined as one requiring psychiatric inpatient attention, as judged by one of our Institution’s staff psychiatrists. Diagnoses that are included in the psychiatric group are bipolar disorder, schizophrenia, major depression with and without psychotic features. The study was approved following Research Ethical Board review.
We retrospectively analyzed a group of 168 patients with a primary psychiatric diagnosis that were hospitalized at Nicklaus Children’s Hospital, Miami, Florida between the years 2010 and 2017 and who had an fMRI performed due to psychiatric symptoms. Patients were diagnosed by a psychiatrist of the hospital staff. The diagnoses were either confirmed at admission or while on the inpatient floor. Symptoms experienced included hallucinations, delusions, aggressive behavior, mood dysregulation and suicidal ideation. In this study, participants were included based on the following criteria: (1) Patient’s primary diagnosis is psychiatric; (2) Patient was experiencing hallucinations delusions or mood instability at the time of the imaging; (3) Patient’s age was between 6 and 17 years of age. Exclusion criteria included: (1) history of epilepsy; (2) current or previous brain lesion or neurosurgical intervention; (3) incomplete results from movement or artifact noise; (4) history of substance use disorder. The patients had a mean age of 13.36 with a range from 6 to 17-years-old at the time the imaging was done. Of the 213 initial patients, 168 were included in this study. These patients had a mean age of 13.45 with a range from 6 to 17-years-old at the time the imaging was done. Forty-five patients were excluded from the study due to concurrent brain lesion, history of epilepsy, or other systemic condition. The patients were further categorized by sex and handedness.
| Table 1. Demographic Characteristics of the Sample |
|
Control
|
Psychiatric
|
| Variable |
| N (initial) |
104
|
213
|
| N |
75
|
168
|
| Age mean |
10.99
|
13.45
|
| Age range |
6 to 17
|
6 to 17
|
| Age SD |
3.6
|
3
|
| Male |
35
|
97
|
| Female |
40
|
71
|
| Right Handed |
62
|
151
|
| Left handed |
10
|
15
|
| ambidextrous |
3
|
2
|
| Not determined |
|
2
|
| Sedated |
19
|
48
|
| % Sedated |
0.25
|
0.29
|
N prior to exclusion=N (initial)=317; N included in study=N=243;
Total patients excluded=74 |
As a comparison group, we randomly selected 104 patients in whom a primary indication for imaging was intractable epilepsy in absence of psychiatric symptoms. For this group, the inclusion criteria included: (1) History of intractable epilepsy; (2) Patient age at time of imaging was between 6 to 17-years-old. Exclusion criteria included: (1) concurrent psychiatric diagnosis; (2) neurosurgical intervention or visible lesion on imaging; (3) incomplete results from movement or artifact noise; (4) history of substance use disorder. The mean age of the comparison group was 11.06 with a range of 6 to 17 years of age. Twenty-nine patients were excluded due to age, neurosurgical intervention or lesion. Of the75 patients included, 25.3% required sedation with propofol 100 µg/kg/m. The patients were further categorized by sex and handedness.
M Technique
Patients in both groups had a resting-state fMRI sequence, and a three dimensions (3D) volumetric anatomical magnetic resonance imaging (MRI). All cases were scanned with the same MRI machine, an Intera Philips Medical System 1.5 T MR Scanner (Philips Health Care, Netherlands). Axial T1-weighted MRI 3D volume was acquired for anatomical reference with a field-of-view (FOV) of 240X240 mm, voxel size 1X1X1 mm, 120-176 slices in one slab, and the following acquisition settings: repetition time (TR): 25 ms, echo time (TE): 3.8 ms and flip angle of 30 degrees. The resting state (rs-fMRI) consisted of a blood oxygenation level dependent (BOLD) sensitive sequence with the following settings: 200 time-points, FOV 240 mm, matrix 64X64 voxels (voxel size 3.6X3.6X6.0 mm), 14-21 axial interleaved slices with no gap, TR: 2000 ms, TE: 60 ms, flip angle: 65 degrees, standard shim mode. Patients were instructed to keep their eyes closed and focus their attention on the breath, trying to feel the air flow in the nostrils.
rs-fMRI Processing
For each patient, the rs-fMRI data, was analyzed utilizing the fMRI Melodica tool, from Forecast Systems Laboratory (FSL) 4.1.9.10 The tool provides ICA extraction of spontaneous brain networks. Data was re-aligned, normalized, and spatially smoothed by use of a Gaussian kernel of full width at half maximum of 7 mm; and then band-pass filtered (0.01<f< 0.1). Cases with motion average (x, y, z translational plus 3 rotational) of more than 2 mm were discarded for further processing.
The activation of the amygdala was assessed by one of the authors based on visual inspection, and (1) comparing the patient results with prior established findings9 taken as template, and (2) the Online Brian Atlas Reconciliation Tool, available at http://qnl.bu.edu/obart/explore/AAL/. The amygdala network (as presented in the neural components yield by the ICA) consist of the whole amygdala, the accumbens nucleus, hypothalamus, ventral thalamus and the striatum (Figure 1).
Figure 1. Example Image of Amygdala Activation with Intensity Depicted by Pixel Color
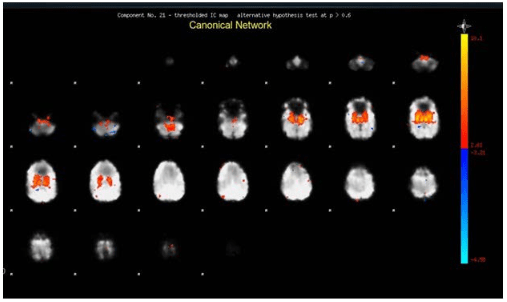
For those patients under sedation, only those who showed activation of the primary visual network were included. Annotations of the melodic tool yield included number of neural components, number of networks judged canonicals, number of networks judged as non-canonicals and a categorical binary determination of the involvement of the amygdala as present or absent. The amplitude of the amygdala activation in each patient was annotated and documented based on the canonical network intensity within the amygdala provided by the tool.
Comparisons
Statistical comparisons of amygdala activation between groups were performed by means of Chi-square (two-tails) test. The comparison between the groups and the effects on amygdala activation of age, gender, handedness, and need for sedation were investigated with 2X2 tables running the Fisher exact test interactions were judged with a threshold of p<0.05.
RESULTS
There were no statistical differences between gender (p=0.11), handedness (p=0.12) and sedation/non-sedation ratio per group (p=0.6). The age group averages differ on our two groups. The psychiatric group is 22.2% older than the epilepsy group (p<0.01). In spite of our effort to minimize age as a variable factor, the patient average in the psychiatric group was higher. We believe the primary reason behind this is the age of onset for pediatric psychiatric diseases. The symptomatology in all included psychiatric diagnoses are more frequently expressed at pre-adolescent/adolescent ages.
The results of our study showed a considerable increase in the frequency of identified networks in the amygdala in psychiatric patients vs the epileptic control group. The average number of unique networks identified in the psychiatric and epileptic group was 11.75 and 12.66 respectively. The percentage of patients with identified networks that show an increase in amygdala activation in the control group was 10.7%. The percentage of patients that show an increase in amygdala network activation in the psychiatric group was 50.0%. The statistical analysis of results can be found on Table 2.
| Table 2. The Statistical Differences Between Both the Epileptic and Psychotic Patient Populations |
|
Control
|
Psychiatric
|
| Neural Networks |
| Range |
4 to 25
|
5 to 28
|
| Average |
11.75
|
12.66
|
| SD |
4.41
|
5.42
|
| Canonical |
| Range |
3 to 22
|
3 to 20
|
| Average |
9.16
|
9.65
|
| SD |
3.88
|
4.64
|
| Non-canonical |
| Range |
0 to 9
|
0 to 12
|
| Average |
2.56
|
3.06
|
| SD |
2
|
2.24
|
| Amygdala activaion |
| N= |
8
|
84
|
| % |
10.7
|
50.0*
|
| Activaion max |
| Mean |
7.73
|
7.34
|
| STDev |
4.97
|
4.8
|
(*)Amygdala Activation Statistical Difference:
Chi square: P=0; Z for 95% CI=1.96; Pearson’s=35.793
Intensity Act: Z score; t-test: 0.876 (95% CI=-3.924<0.3<3.894, Wald) |
The interactions between groups (epilepsy vs psychiatric), condition (sedation) and ICA outcome is shown in Table 3. No interaction was observed between group and number of networks, number of canonicals or non-canonicals in either of the conditions.
The effect of sedation on the ICA yield is shown in Table 4. No significant statistical differences were seen in the epilepsy group. Sedate psychiatric patients show more non-canonical networks as compared to non-sedate individuals in the same group (p=0.035; CI 95%=1.96. CI dif: 0.085<0.97<1.854). The rest the interactions were discarded. The significant difference on the psychiatric patients seems to be dragged by 3 outliers with values beyond 3 standard deviations of the mean.
| Table 4. Statistical Differences of ICA Yield with Respect to Sedation |
|
Group
|
|
| Epilepsy |
p value of Mean Dif |
| Sedate vs Non-sedate |
| #Networks |
0.9
|
| #Canonicals |
0.474
|
| #Non-canonicals |
0.253
|
| Psychiatric |
| Sedate vs Non-sedate |
| #Networks |
0.192
|
| #Canonicals |
0.693
|
| #Non-canonicals |
0.035
|
| Numbers are p values derived from statistical analysis (t-test) from columns in Table 3 |
Numbers are p values derived from statistical analysis (t-test) from columns in Table 3.
| Table 3. Interactions between Group and Sedation for ICA Outcome |
|
Epilepsy
|
Psychiatric
|
|
| Averages
Sedate |
N=
|
SD= |
N= |
SD= |
p=
|
|
19
|
|
48
|
|
|
| #Networks |
11.63
|
4.78 |
17.4 |
5.53 |
0.17
|
| Averg canonicals |
8.53
|
4.74 |
13.72 |
4.79 |
0.3
|
| Averg non-canonicals |
3.05
|
2.22 |
3.7 |
2.73 |
0.29
|
| Non-sedate |
56
|
|
120 |
|
|
| #Networks |
11.79
|
4.33 |
11 |
4.34 |
0.51
|
| Averg canonicals |
9.38
|
3.57 |
8.23 |
3.68 |
0.78
|
| Averg non-canonicals |
2.39
|
1.91 |
2.84 |
2.01 |
0.21
|
The effect of sedation in amygdala activation is summarized in Table 5. There was more frequent activation of the amygdala seen in the psychiatric group under sedation (p=0.032).
| Table 5. Effect of Sedation in Amygdala Activation |
| Amygdal Interactions |
Epilepsy
|
Psychiatric
|
| Sedated |
| N= |
19
|
48
|
| #Act |
2
|
19
|
| Non-sedated |
| N= |
56
|
120
|
| #Act |
6
|
65
|
| (Fisher exact test) |
0.325
|
0.032
|
DISCUSSION
The present rs-fMRI study supports the hypothesis that children with severe psychiatric conditions have a basal amygdala activation when compared to a similar population with intractable epilepsy. The epilepsy group showed amygdala activation at a much lower rate (10%). This rate of activation is congruent with matching age normal subjects from observational data previously obtained.9 To the best of our knowledge, this is the first publication of this finding utilizing rs-fMRI based on ICA in the pediatric population.
Core to the major psychiatric conditions including psychosis, aggressive behavior, and delusions is the dysregulation of emotion. The involvement of the amygdala in emotional processing seems well established. A PubMed search utilizing the words “amygdala” and “emotion” yields 8808 results (search date: 05/10/2018). But what do we mean by “processing emotion”? There is probably not a single response for this question. Most modern approaches see the amygdala at the center of processing responses related to the “most significant objectives of the subject.” According to Pessoa L, et al11 in doing so, the amygdala may evaluate “what is it?” and “what to be done?” These questions result in the construction of emotions in a process intertwined with cognition.
Our study findings are congruent with previous evidence that show an abnormal level of amygdala activity in the presence of psychosis and/or mood dysregulation. Anatomical and functional abnormalities in the amygdala of psychotic patients have been noted across modalities including MRI,12,13 and positron emission tomography (PET)14 studies that consistently show altered connectivity patters and activation compared to controls. The findings are, however, disparate, suggesting a complex interplay of different subject-related variables. For example, different expression of a gene related to serotonin transporter correlates with amygdala activation in response to fearful and happy faces.15 In a study of patients with intermittent explosive disorder, the left amygdala responded greater to angry faces in an fMRI study as compared to normal subjects and patients with the same condition but having history of alcohol abuse.16 A 2016 study found estrogen levels in women seem to modify the amygdala connectivity in women.17 Patients with depression have less amygdala activation on fMRI involving negative valence stimulus as compared to healthy controls.18 In a group of adolescents, responses to negative valence distractors revealed increased amygdala activation with respect to healthy controls.19 Adolescents with bipolar disorders seem to have lower threshold for amygdala activation from emotional face processing in fMRI when compared to control subjects.20 Another group of adolescents with anxiety disorders showed greater amygdala activation than matching controls, in a fMRI study in which a social preference task was given.21
Not in all studies relating amygdala activation or connectivity with major psychiatric disorders show hyperactivation. Decreased functional connectivity in rs-fMR between the amygdala and the frontobasal areas has also being informed in a group of patients with major depressive syndrome.22 Also, in a review of the literature it has been found that the task-related amygdala activation, and amygdala connectivity are diminished in patients with schizophrenia.23 Although we focused our attention in the amygdala activation, independent components often have shown a ganglio-basal connectivity. Indeed, as shown in Figure 1, amygdala activation extends dorsally including areas of the ventral striatum (including areas of the nucleus accumbens) and the caudate nucleus. Medial structures most likely related to mammillary bodies and hypothalamus were also seen involved. Interestingly, we did not find connectivity to the basal aspect of the frontal lobes, cingulate, insula and preseptal areas as frequently described in other studies or rs-fMR and amygdala activation in psychiatric patients with bipolar disorder, depression.24,25,26,27
Our connectivity findings are based on independent component analysis and not in ROI-based connectivity as seen in most of the studies referenced above. Similar ICA methods used in adults with cocaine-user disorder showed amygdala connectivity to putamen, pallidum, caudate, thalamus and hippocampal areas.28 This pattern of activation is greater in patients with history of trauma.
The complex and multifactorial interactions that mediate amygdala function in emotion processing and response may obscure our understanding. Nevertheless, all the previously mentioned studies and our own results are concordant with the core role this structure has in conditions where emotional regulation is impaired.
Pitfalls
This study has some potential shortcomings. First, there was a statistically significant difference of age between the epilepsy and psychiatric groups. This has a clear explanation. The age of onset for schizophrenia and or mood disorders with associated psychosis tend to skew the patient average age towards late adolescence, while intractable epilepsy are usually related to congenital anomalies that are manifesting early on life and that require early intervention. This explains the discrepancy and the difficulty to match the sample. However, we feel that both groups represent cohorts of adolescent and pre-adolescent children with similar neuro developmental maturation. Moreover, the frequency of amygdala activation found in the epilepsy group is the same than the frequency found in the normative group whose age mean (12 years, SD 4.2) was in between than those of our groups.
Another potential shortcoming is the need for sedation in a significant percentage of both the groups. Interactions of sedation were found only in the psychiatric group. Within this group, sedated patients showed more non-canonical than canonical components and more amygdala activation. It is difficult to speculate about these findings with just the results of this study. Although the doses of sedative were parallel between the groups, the epilepsy patients had the rs-fMRI series about 15 minutes into the session (after the task-related fMRI), while the psychiatric patients had this within the first 5 minutes. The effect of this difference, if any, is not known. We did not measure the blood levels of sedation. However, we made a physiological threshold of brain “reactivity”, assuming one of the most stable networks, the primary visual, was present. Each case that required sedation was kept within a narrow window of sedation, enough to keep still, but not to the point of alteration the response of this network. The persistence of the amygdala network even under sedation suggests an ongoing primary phenomenon that put the finding at the origin and not at the consequence level and basically discards the effect of the environmental stressful features of the MRI exam.
For the purposes of this study, patient populations were grouped into two rather broad groups without further specifiers. Patients from the psychiatric group potentially had different diagnosis and or severity of symptoms at the time of imaging. The control group, although at a minimum had intractable epilepsy also were not sub-classified by diagnosis or severity.
Medication management was not taken in to account in the study and would also need to be addressed in future research. Many of the patients from the psychiatric group were on some form on antipsychotic treatment at the time the imaging was done. Anticonvulsants were used sparingly across both groups for epilepsy or mood stabilization. The psychiatric diagnoses themselves are based solely on clinical judgment at the time of the hospitalization and in some cases with limited patient history. The possibility of misdiagnoses cannot be excluded.
We did not analyze the effects of substance abuse, Attention deficit hyperactivity disorder (ADHD) or autism spectrum disorder. We acknowledge the difficulty to factor these important confounds. We were able to exclude known atrial septal defect (ASD), and substance use that were recorded as part of medical records but these confounders cannot be completely ruled out. However, to elucidate their impact will require a better understanding of the findings and a much larger number of subjects allowing meaningful stratification groups. Interestingly the same comorbidities, although likely in less degree, are associated with epilepsy.
In the psychiatric group whom met criteria for psychotic symptoms due to hallucination, the nature of the hallucinations was not taken into account. Much of auditory and visual hallucinations in schizophrenic patients are negative, evil or carry a negative connotation. This negative emotional valence could be further classified and correlations made with amygdala activation in these patients that may experience fear vs those with positive emotional response to the hallucinations.
Our findings require further development and refinement to ascertain which specific etiologies or symptoms are more related to the finding. This may ultimately evolve into a biomarker for a specific psychiatric condition with potential usage to guide treatment. Our aim was to demonstrate group differences on resting-state networks involving the amygdala. We did not strive to correlate the findings with state, traits, trajectories or treatment outcome. We are planning future studies to peruse that path.
CONCLUSION
In conclusion, we found a statistically significant increase in amygdala activation as seen on fMRI in the pediatric population with a positive history of psychosis. This may represent a heightened response to emotional stimuli caused by paranoia and or hallucinations. It could also be a compensatory mechanism by which increased perfusion to these affected areas result of a functional impairment from the underlying neuropathology.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.






