INTRODUCTION
In Cuba, tumors of the Central Nervous System (CNS) for children and adolescents account between 18 and 20% of all tumors in this group of age.1,2,3 The main methods of treatment are surgery, radiotherapy, and chemotherapy.4 Radiation therapy is a major treatment avenue in medulloblastomas, primitive neuroectodermal tumor (PNET) in some cases of germinomas consist of craneospinal irradiation (CS ), and a supplementary boost to the post-operative tumor bed, followed by chemotherapy. In diffuse infiltrative brainstem gliomas (DIPG) local radiotherapy is applied, although in our institute it is combined with a monoclonal antibody (Nimotuzumab), which allows up to a 33 % expected survival for as long as 10-years.5 Ependymomas receive only local irradiation plus chemotherapy, except in cases of extension to the spinal canal, where they received CS. Astrocytomas and glioblastomas only receive local irradiation and in some cases chemotherapy.
The irradiation in CNS tumors in the pediatric ages is associated with damage to uninvolved tissues, which may negatively effects cerebrovascular functions, neurotoxic, neurocognitive, endocrine, psychosocial and the quality of life.6,7 In patients with recurrent tumors8 reirradiation has been proposed as a modality of treatment. This reirradiation could be associated with the risk of injury9 in varying degrees. Thus it is necessary to weigh benefits versus toxicity. In order to offer benefits, newer approaches in radiation therapy are employed, as intensity-modulated radiation therapy (IMRT), stereotactic radiosurgery and radiation therapy with particles and so on.10 The present study is a report for a series of 17 children and adolescents who received reirradiation for relapse of the primary tumor.
MATERIALS AND METHODS
This is a retrospective, observational, non-randomized study of a series of 17 children and adolescents, irradiated at the Instituto Nacional de Oncologia y Radiobiologia, in Havana, Cuba. The patients were treated for the first time between the period of January 1999 and August 2016, with a periodical evaluation until November 2018, or to the time of death. The intention of reirradiation was chosen according to symptoms, prior treatment history and especially in the interest of patients, family and the referring physician. In all cases, a consent form was obtained. The dates for analysis were obtained from the medical records and included data of primary irradiation, data of relapse and reirradiation, as well as information regarding the irradiation doses, data of last follow-up, disease status, and date of death. Overall survival function was estimated by the Kaplan-Meier method.
Initial treatment consisted of surgery, when possible (biopsy or partial or gross resection), and then the first course of radiotherapy, and chemotherapy, if indicated. In all patients, reirradiation was accomplished with a linear accelerator (LINAC) machine, planed in three dimensional (3D) in 2 cases IMRT and with image-guided radiation therapy (IGRT) in 3 cases, with a daily dose of 1.8 Gy, 5 sessions per week of treatment. For two patients with diffuse intrinsic pontine gliomas (DIPG), the irradiation was combined with the monoclonal antibody, Nimotuzumab. The 8 patients with the diagnosis of medulloblastoma received a CS dose of 23.4 Gy in the first irradiation, two of them were reirradiated in CS with 20 Gy, and all with a dose of 46 Gy in tumoral volume. Germinomas received 23 Gy in CS in one patient and 30 in tumor, and in reirradiation 46 Gy in the tumor. Astrocytomas grade III received between 56 and 59.4 Gy, and 54 to 56 Gy in reirradiation. One patient with the diagnosis of ependymoma received the first dose of 54.4 Gy in the tumor and CS dose of 19.6 Gy and 44 Gy in the tumor during the second course of irradiation. Finally, one patient with the diagnosis of oligodendroglioma received 54 Gy in the first irradiation and 54 Gy in the second.
| Table 1. Characteristics of the Reirradiated.Patients |
| Id |
Sex |
Diagnosis |
Time-1 (Months) |
Dose Reirradiation (Gy) |
Cumulative Dose (Gy) |
Current Status |
Tos (Months) |
| 1 |
M |
Brainste m |
44 |
54 |
90,0 |
Alive |
90 |
| 2 |
F |
Brainste m |
21 |
34 |
88,2 |
Dead |
14,4 |
| 3 |
M |
Medulloblastoma |
18 |
46 |
80,0 21,0 CS |
Dead |
10,8 |
| 4 |
M |
Medulloblastoma |
89 |
46,0 |
100,0 23,0 CS |
Dead |
18,0 |
| 5 |
M |
Medulloblastoma |
104 |
50,0 |
104,0 23,0 CS |
Dead |
18,0 |
| 6 |
F |
Medulloblastoma |
131,0 |
50,0 |
104,0 23,0 CS |
Dead |
12,0 |
| 7 |
F |
Medulloblastoma |
56,0 |
45,0 23,0 CS |
99,0 43,0 CS |
Dead |
20,4 |
| 8 |
M |
Medulloblastoma |
15,0 |
52,2 |
106,0 23,0 CS |
Alive |
48,0 |
| 9 |
M |
Medulloblastoma |
83,0 |
54,0 20,0 CS |
108,0 43,0 CS |
Alive |
26,4 |
| 10 |
M |
Medulloblastoma |
34,0 |
54,0 |
108,0 23,0 CS |
Alive |
42,0 |
| 11 |
F |
Germi1oma |
83,0 |
44,0 23, o CS |
76,0 23,0 CS |
Dead |
14,4 |
| 12 |
F |
Germi1oma |
32,0 |
30,0 |
76,0 |
Dead |
9,6 |
| 13 |
M |
Anaplastic Astrocytoma |
27,0 |
54,0 |
111,4 |
Dead |
8,4 |
| 14 |
F |
Astrocytoma GIll |
119,0 |
57,0 |
114,6 |
Dead |
9,6 |
| 15 |
M |
Astrocytoma GIll |
22,0 |
56,6 |
112,6 |
Dead |
1,3 |
| 16 |
M |
Ependymoma |
12,0 |
42,0 |
96,0 |
Alive |
8,4 |
| 17 |
M |
Oligodendroma |
109,0 |
54,0 |
108,0 |
Dead |
14,4 |
| Time-1: Time between first and second irradiation; CS: Craneospinal irradiation; TOS: Tine of survivalafter reirradiation |
Twelve patients were treated with surgery at the time of recurrence, which ranged from the only biopsy to partial resection. Twelve received also chemotherapy and 3 monoclonal antibodies, according to the treatment plan in our department.11
RESULTS
Seventeen patients were included in this series: 11 male and 6 female, with a median age at the time of the reirradiation of 14.0-years CI 95%.12,13,14,15,16,17
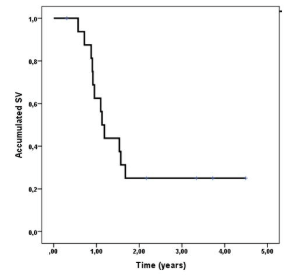
Among those reirradiated the median of time of disease relapse was 8 to 9-years from the first to the second irradiation. Four patients received CS plus tumor volume reirradiation, while the rest only to the primary site of recurrence. Five patients are alive with a follow-up year range between 8 and 1-year. Median total dose received with both irradiations was of 100 Gy IC 95% (94.2- 108 Gy). The estimated survival rate was 62.5% at 1-year and 25% at 2-years (Figure 1), with a median survival time of 1.13-years CI 95% (0.96-1.30)
Figure 1. Overall Survival Since Time of Reirradiation
Eight patients with medulloblastoma, constituting the 47.1% of cases, with a survival rate of 37.5% at 2-years compared with 12.5% for other tumors and a median survival time 1.57-years CI 95%(1.38-1.76) compared with 0.92-years CI 95%(0.65-1.19) for other tumors. There was not a statistically significant difference between the two groups (p=0.072) (Figure 2).
Figure 2. Survival Functions from Re-Irradiation According to Histology of Tumors
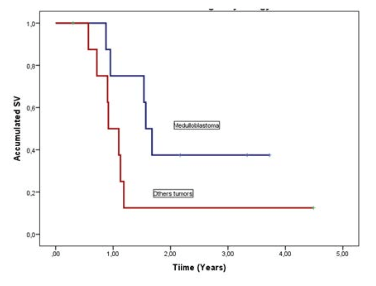
All the relapse cases who not received reirradiation died between 3-months to one year.
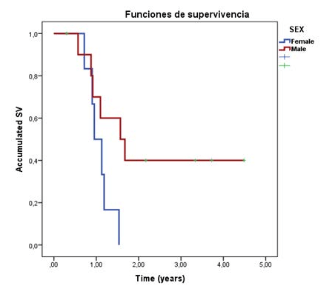
All surviving patients were male (2-year survival, 5 in total, 40%), with a median estimation of 1.57-years CI 95% (0.67-2.46)]; Female patients median survival time estimation was 0.953-years [CI 95% (0.6-1.22)]. Median survival time according to gender is significant in the clinical response, although there was no statistically significant difference between the groups (p=0.059) (Figure 3).
Figure 3. Survival Functions from Reirradiation According to Sex
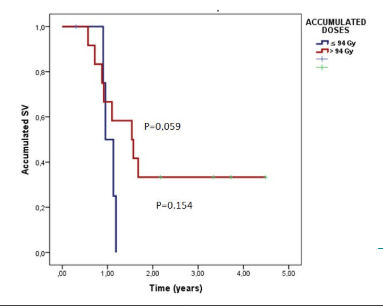
The survival for 2-years percentage for the patients who received >94 Gy was 33% and their median survival time was 1.09- years [CI 95% (0.57-1.63)]. This was higher than the survival for 2-years percentage for the patients who received ≤94 Gy (14.3%). The latter group had a median survival time of 1.19 [CI 95% (1.04-1.33)]. However, the difference between the two groups was not statistically significant (p=0.154) (Figure 4).
Figure 4. Survival Functions from Reirradiation According to Total Accumulated Dose
We found preserved cognitive function in surviving patients, especially over 12-years of age; 3 had a Karnofsky-Lansky (K-L) scale of 100%, 1 had a K-L of 90% and 1 had a K-L of 70%.
DISCUSSION
The management of CNS tumors in children and adolescents include the use of surgery, and/or irradiation, chemotherapy, and monoclonal antibody.5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29 A response is frequent, nevertheless long-term survival is observed between 20% to 80% of those patients.11,17,19 During their evolution, there are relapses, that could occur a few months to years after the first irradiation.9,13,14,15
Rao et al8 reported reirradiation in 67 children with recurrent CNS malignancies and found that the median overall survival after the completion of repeated radiation therapy (RT) was 12.8-months for the entire cohort and concluded that CNS reirradiation could be a reasonable treatment option. In our study, a similar range of median overall survival was obtained (12.2-months).
Several studied reported positive outcomes for reirradiation. Wetmore et al21 reported 14 patients with recurrent medulloblastoma, with 6 still alive with a median follow-up of 12.1-years (range 7.2 -14.6-years) and concluded that the use of reirradiation may prolong survival, but that the expected 2-year overall survival after disease progression is less than 25%. In our study, we had 8 patients with recidivant medulloblastomas, with 3 still alive (37.5%). In an overview of treatment options for recurrent ependymoma and medulloblastoma, Nieder et al24 concluded that reirradiation was associated with a better outcome. In reirradiation of recurrent CNS tumors in children, Chang A et al26 reported a median follow-up time for survivors of 97.4-months, with 5 of 11 being alive. Lobon-Iglesias MJ et al27 found an improved survival of 7.5 versus 4-months with a second course of radiotherapy in diffuse intrinsic pontine glioma (DIPG). In addition, Waxweiler T et al29 reported the use of hypofractionated irradiation in 23 patients, with 3 or 5 fractions, with a median tumor stabilization of 10.5-months.
With the reirradiation, we evaluate the possibilities of improving survival. The dose of reirradiation in our study was not always the same for all cases and was based on clinical factors and previous dose received. The eight patients reirradiated for medulloblastoma receive 37.5% of survival, better survival compared with the rest of patients. There has been reported complications in the post radiation and reirradiation evolution4,7,8,23 nevertheless we did not found significant complications in our patients, as all deaths were due to a progression of the primary tumor.
There has been a debate about the use of reirradiation at the time of relapse because of possible improvement of survival, however, this can be associated with higher toxicity.9,23 Perhaps the side effects that are associated with reirradiation should be overlooked since the risk of death by the progression of the tumor is high for recurrences. Reirradiation could increase the time of survival, and amelioration of quality of life, which are very important factors for patients and parents. Even a third course of radiation therapy had been reported Tsang D et al25 and may be helpful in selected cases of ependymomas, for local control and palliation.
CONCLUSION
In this series, reirradiation is an option for the treatment of recurrence of CNS tumors, in order to increase the time of survival, even palliation, with good cognitive functions.
IRB APPROVAL
This study has been approved by the Ethical Committee of Institute of Oncology.
CONFLICTS OF INTEREST
The authors report no conflicts of interest.









