There is limited data comparing fluids used for continuous renal replacement therapy (RRT) in the treatment of acute kidney injury. We undertook a retrospective observational study and identified that when the standard RRT fluid used in our institution was changed from a phosphate and potassium free fluid to a potassium and phosphate containing fluid, patients spent a significantly greater proportion of therapy time with hypocalcaemia, hyperphosphataemia and metabolic acidaemia. Our data suggest that phosphate containing fluids may not be ideal for unselected patients with acute kidney injury and their use may explain persistent acidaemia, and derangement of serum calcium and phosphate.
INTRODUCTION
Acute kidney injury (AKI) is increasingly common in critically ill patients and associated with significant morbidity and mortality.1 One large multicenter study showed that over 16% of critically ill patients are diagnosed with AKI within forty-eight hours of ICU admission with a 22% overall incidence of AKI during ICU admission, as defined by the (risk, injury, failure, loss of kidney function, and end-stage kidney disease) RIFLE classification.2 AKI is an independent risk factor for death.3 Inhospital mortality for patients with AKI exceeds 25%4 and mortality amongst ICU patients requiring renal replacement therapy approaches 70%.5
Continuous renal replacement therapy (CRRT) is the favored treatment modality for AKI in critically ill patients in Australasia. Many units, including ours, use Hemosol-B0 as their standard CRRT fluid.5 Hemosol-B0 does not contain PO43- or K+, leaving patients susceptible to hypophosphataemia and hypokalaemia unless plasma levels ([PO43-]pl and [K+]pl) are assiduously monitored and maintained.6 Depending on CRRT intensity and duration the incidence of hypophosphataemia with CRRT can exceed 65%.
Phosphate is required to form the high-energy bond that provides the major energy currency required for metabolism. Complications of hypophosphatemia include respiratory muscle dysfunction and prolonged respiratory failure,7 cardiac dysrhythmia (particularly ventricular tachycardia), reduced myocardial contractility and neuromuscular depression. Longer term, hypophosphataemia is associated with delayed recovery from AKI and higher incidence of chronic renal failure one year after dialysis commencement.8 Hypophosphataemia is independently associated with worse ICU survival; the duration of hypophosphataemia predicts mortality.9
Phoxilium is a phosphate and potassium containing CRRT fluid, introduced to mitigate the risk of hypophosphataemia and need for potassium supplementation.10 The composition of Hemosol-B0 and Phoxilium are compared in Table 1. Concerns regarding the use of Phoxilium as a standard RRT fluid include (i) metabolic acidosis promoted by the lower [HCO03–] and [lactate], and increased [HPO42-] in Phoxilium, and (ii) suppression of ionized plasma [Ca2+] (i[Ca2+]pl) as a consequence of increased plasma [PO4–] and low [Ca2+] in Phoxilium.
Table 1: Composition of RRT fluids (mmol/l).
|
Hemosol-B0 |
Phoxilium |
| Phosphate |
0 |
1.2 |
| Potassium |
0 |
4 |
| Bicarbonate |
32 |
30 |
| Lactate |
3 |
0 |
| Calcium |
1.75 |
1.25 |
| Magnesium |
0.5 |
0.6 |
| Sodium |
140 |
140 |
| Chloride |
109.5 |
115.9 |
Previous work identified that after administering Phoxilium for 42 hours to selected patients median [HCO3–]pl and i[Ca2+]pl were reduced but [PO43-]pl maintained relative to a matched cohort who continued to receive Hemosol-B0.10 It is common practice in most intensive care units to identify a default fluid to be used for CRRT. The biochemical consequences of replacing Hemosol-B0 with Phoxilium as the default CRRT fluid to an unselected patient cohort, and the potential benefit of minimizing handling of concentrated K+ solutions (identified as a high risk intervention by organizations in Australia, Canada, the US and UK11,12), remain unknown.
We undertook a retrospective observational cohort study to investigate the hypothesis that using Phoxilium, rather than Hemosol-B0, as a standard CRRT fluid would be associated with more normal and consistent phosphate levels and lower requirements for non-RRT electrolyte supplementation. The primary study end-point was the proportion of RRT duration spent with plasma [PO43-] within the normal range. Secondary analysis were performed to identify (i) differences in the proportion of the RRT period spent with [HCO3–]pl and i[Ca2+]pl in the normal range and (ii) differences in the requirement for non-RRT fluid K+ and PO43- supplementation.
METHODS
Study Population
Critically ill patients over 18 years old admitted to the Royal Adelaide Hospital Intensive Care Unit between October 2011 and October 2012 who required renal replacement therapy were recruited into the study.
Intervention
Patients underwent CRRT with continuous veno-venous haemodiafiltration (CVVHDF) with 40 ml/kg/hr effluent rate. This relatively high RRT dose was used to ensure minimum achieved doses of 25 mls/kg/hr (previous local audit data identified that patients prescribed CRRT receive CRRT for an average 16 hours/day). Two six-month treatment periods were compared: during the first period, from October 2011 to March 2012, the standard renal replacement fluid was Hemosol-B0, (HemosolB0 standard period) with K+ added to RRT fluid bags at the clinicians discretion. During the second period, from May 2012 to October 2012, Phoxilium was the default CVVHDF fluid (Phoxilium standard period). Both fluids were manufactured by Gambro, Lundia AB, Lund, Sweden. Patients receiving RRT fluid other than the designated default for that period were excluded from data analysis. The study was performed with local ethics committee approval.
Data Collection
Demographic, clinical and outcome data were recorded and mean delivered dialysis dose calculated. Daily plasma [PO43-]pl, i[Ca2+]pl, [HCO3–]pl, [K+]pl and [Mg2+]pl results were documented throughout the duration of RRT. Supplementation with K+ and PO43- was recorded. Normal ranges refer to those used by the SA Pathology laboratory (SA Pathology, Rundle Mall, Adelaide, SA 5000, Australia) who were responsible for blood chemistry analysis during the study period.
Data Analysis
Categorical variables are expressed as number (percentage) and compared using Chi-squared or Fisher’s exact test as indicated. Continuous variables are expressed as mean and standard deviation (SD) or when non-normally distributed as median (interquartile range), with between group comparisons performed by t-test or Wilcoxon rank-sum test. A two-tailed p value <0.05 was considered statistically significant. All analysis were undertaken using Stata/MP 14.0, Stata Corp LP software.
RESULTS
There were 86 patients admitted to the Royal Adelaide Hospital Intensive Care Unit between October 2011 and 2012 requiring RRT. Of these 7 patients were excluded from the Hemosol-B0 group and 4 from the Phoxilium due to unavailability of dialysis records or absent biochemical data. A further 9 patients were excluded as they received Hemosol-B0 during the Phoxilium standard period; no patients received Phoxilium during the Hemosol-B0 period as Phoxilium was not available. Data was collected on 35 patients in the Hemosol-B0 group and 31 patients in the Phoxilium group.
There was no significant difference in patient demographics, presence of chronic kidney disease, illness severity, length of stay, time ventilated or hospital mortality between the study groups (Table 2). Initial electrolytes prior to CRRT were not statistically different. There were no significant differences in the aetiology of AKI between the groups, in both groups sepsis was the most common precipitant (51% in the Hemosol-B0 group vs. 57% in the Phoxilium group) followed by cardiogenic shock, hypovolaemia and drug toxicity. There was no betweengroup difference in the cumulative insulin dose administered whilst receiving CRRT (p=0.591).
Table 2: Patient clinical and outcome data: median (IQR) unless otherwise stated.
|
Hemosol – BO |
Phoxilium |
p value |
| Age (years) |
59.2 (54-67) |
59.5 (46-70) |
0.81 |
| Gender – number % male |
21 (54) |
18 (58) |
0.72 |
| APACHE II |
27.5 (23-33.75) |
26.5 (20.25-31) |
0.50 |
| Pre-existing renal disease number (%) |
13 (33.3) |
12 (38.7) |
0.64 |
| Hours receiving RRT |
50 (23-89) |
45 (34-105) |
0.69 |
| CRRT dose (ml/kg/hr) |
37.8 (37.8-39.63) |
38.7 (38.7-39.95) |
0.87 |
| ICU length of stay median (hours) |
205 (74-684) |
155 (70-235) |
0.47 |
| Ventilated days post dialysis initiation – median |
4 (1-12) |
3 (0-5) |
0.19 |
| Hospital mortality – number (%) |
24 (61.5) |
21 (67.7) |
0.59 |
Plasma biochemistry values during CRRT administration are illustrated in Figure 1. For clarity, only the first 5 days are included in the figure since only eight patients in each group underwent more than 5 days of CRRT. Data for the entire duration of CRRT is included in the data analysis.
Figure 1: Median and interquartile ranges of plasma phosphate, calcium, bicarbonate, potassium, and magnesium concentrations over the first five days of renal replacement therapy with either Hemosol-B0 (black circles) or Phoxilium (grey circles). Since only eight patients in each group underwent >5 days of RRT, their data has been omitted from the figure for clarity. Their data is, however, included in all statistical analysis.
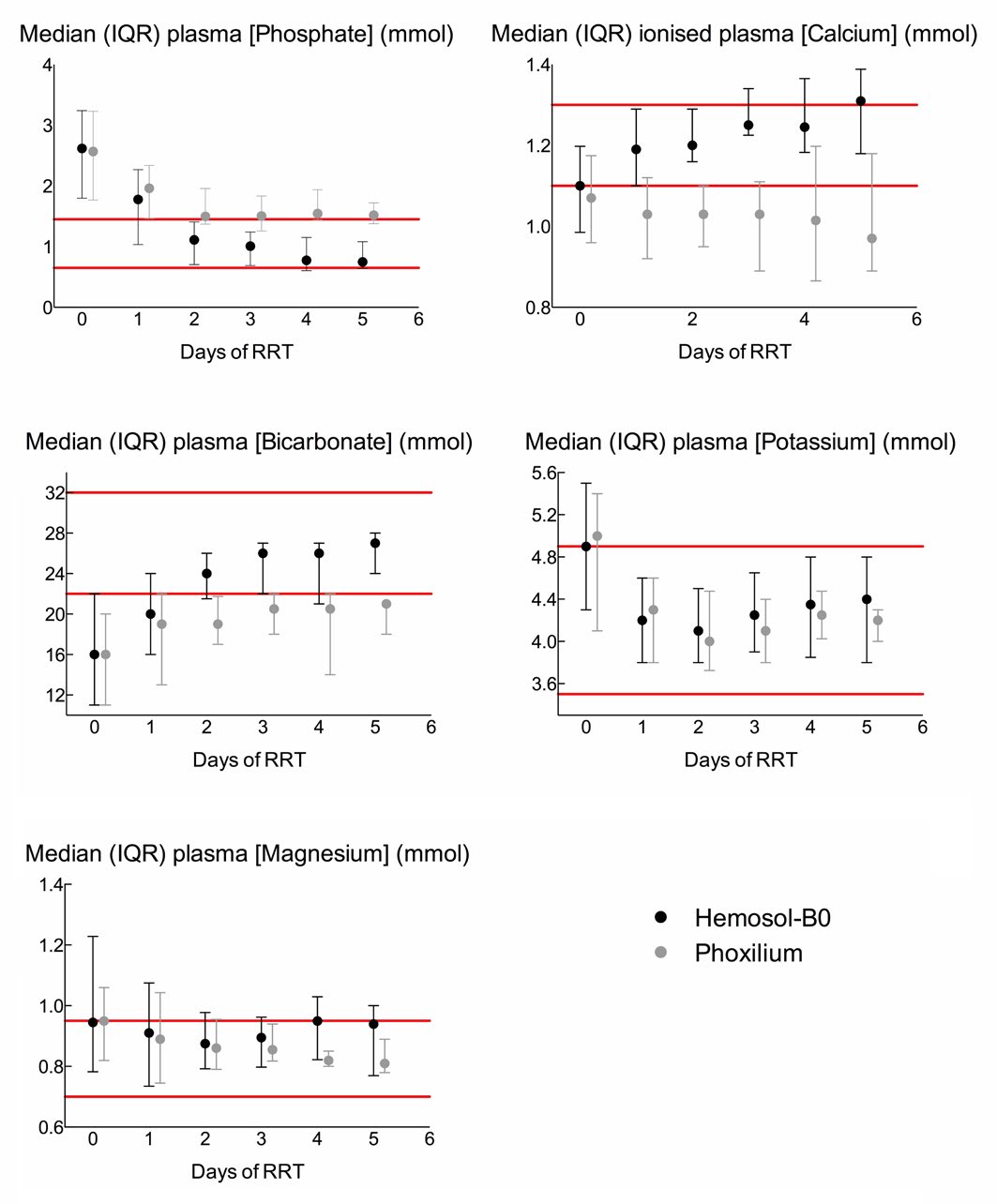
Phosphate
Compared to patients receiving CRRT with Hemosol-B0, Phoxilium recipients spent a greater proportion of CRRT duration with [PO43-]pl outside the normal range (Hemosol-B0 45.3% vs. Phoxilium 66.9% of patient days; p<0.001). In the Phoxilium group the median [PO43-]pl exceeded the upper limit of the normal range on days 2-5 (Normal range: 0.65-1.45 mmol/l).
Ionised Calcium
Patients in the Phoxilium group spent more time with i [Ca2+]pl below the normal range than patients receiving Hemosol-B0 (Hemosol–B0 45% vs. Phoxilium 77.7% of patient days; p<0.001). (Normal range: 1.1-1.3 mmol/l).
Standard Bicarbonate
In the Phoxilium group [HCO3–]pl was abnormally low for a greater proportion of time than in the Hemosol-B0 group (Hemosol-B0 26% vs. Phoxilium 68.5% of patient days; p<0.001). Starting from similar initial values, median [HCO3–]pl remained low on day 1 in both groups; however, median [HCO3–]pl normalized on days 2-5 in the Hemosol-B0 group whilst remaining low in the Phoxilium group. (Normal range: 22-32 mmol/l).
Potassium and Magnesium
There was no difference between the groups in the proportion of RRT duration spent with [K+]pl or [Mg2+]pl in the normal range (Hemosol-B0 85.8% and 61.8% vs. Phoxilium 89.4% and 73.1% of patient days; p=0.30 and 0.27 respectively); in both groups median [K+] and [Mg2+] were normal on each day of CRRT. (Normal ranges: K+ 3.5-4.9 mmol, Mg2+ 0.7-0.95 mmol/l).
Phosphate and Potassium Supplementation
Phoxilium use abrogated the need for phosphate supplementation – no patients in the Phoxilium group required phosphate supplementation whilst 26% of patients in the Haemosol-B0 group required PO43- supplementation. KCl was added to Hemosol-B0 in all but four of the Hemosol-B0 group. In the Phoxilium group, 45% of patients required non-RRT fluid potassium supplementation vs. 41% of patients receiving Hemosol-B0 (p>0.05).
DISCUSSION
When used as a standard CRRT fluid in a tertiary metropolitan intensive care unit, treating AKI with CRRT using Phoxilium, compared to Hemosol-B0, was associated with a greater proportion of treatment time spent with abnormally high [PO43-]pl, low ionized [Ca2+]pl and low [HCO3–]pl. Phoxilium use reduced the need for phosphate but not non-RRT fluid potassium supplementation.
Phoxilium use was associated with a significant period of time spent with [PO43-]pl above the normal range. The median [PO43-]pl was minimally elevated and mild hyperphosphataemia is generally well-tolerated in the short-term, although likely to contribute to i[Ca2+]pl suppression and promotion of metabolic acidaemia, both deleterious to the critically ill. Chronic hyperphosphataemia is associated with cardiovascular risk and mortality in end-stage renal failure13,14; further study is needed to identify whether analogous processes occur during short-term hyperphosphataemia.
Low [Ca2+] and increased [PO43-] in Phoxilium contribute to lowering i[Ca2+]pl. Potential consequences include vasopressor resistance, decreased myocardial performance, arrhythmia, neuromuscular dysfunction and impaired coagulation.15,16 Patients in our study were critically ill, as indicated by their Acute Physiology and Chronic Health Evaluation II (APACHE II) scores, and even mild manifestations of these complications would be deleterious.
Potential mechanisms by which Phoxilium might suppress [HCO3–]pl include: (i) lower [HCO3–] and absent lactate (the metabolism of which consumes hydrogen ions) relative to Hemosol-B0 (ii) relatively high [HPO42-]pl in patients receiving Phoxilium.17 Consequences of acidaemia in vivo are complex18; acidaemia is associated with impaired myocardial performance, arrhythmia and vasopressor insensitivity as well as altered drug pharmacology and immune and metabolic dysregulation.19 Again, given the severity of illness of our patient population, these complications are poorly tolerated.
Although Phoxilium contains K+, its use in preference to Hemosol-B0 did not reduce the need for non-RRT fluid K+ supplementation. This suggests that a policy of adding K+ to RRT fluid at clinicians discretion is at least as effective at maintaining [K+]pl as using RRT fluid containing KCl as standard. Indeed two potential advantages of an RRT fluid not containing KCl as standard are (i) use in hyperkalaemic patients (the probable reason for 9 patients in the Phoxilium period receiving Hemosol-B0) and (ii) the option to use a potassium salt with a weak anion such as acetate instead of chloride. Nevertheless, Hemosol-B0 requires more handling of KCl concentrate when supplementation to the RRT fluid is considered. Hospital surveillance systems identify KCl administration as the most frequent source of fatal drug errors11; guidelines recommend limiting KCl availability in clinical areas, and storage in a locked cupboard with meticulous (and time consuming) safety check procedures in place prior to its use.20
Using Phoxillium removed the need for phosphate supplementation. Our data are consistent with the important data published by Chua et al10 that identified metabolic acidosis and hypocalcaemia amongst selected patients receiving CRRT in whom Phoxilium had been introduced to at clinicians discretion. However, in contrast to their finding that plasma [PO43-] was better maintained in patients receiving Phoxilium, we found that that the use of Phoxilium was associated with a greater proportion of time spent outside the normal plasma range of [PO43-]. The difference can be explained by the different way in which the RRT fluids were used. In our study, the RRT fluids were used as unit defaults rather than at the discretion of the treating consultant (although clinicians could actively choose to use either fluid). This reflects common practice in many intensive care units, whereby a default CRRT fluid is identified, with the option to select an alternative at clinician’s discretion. Notably, the default fluid was used in 89% of cases underlining the importance of ‘default’ clinical pathways and suggesting limited selection bias.
The use of potassium and phosphate containing renal replacement fluids in patients with AKI with hyperkalaemia and hyperphosphataemia is potentially harmful. In our study 9 of the Phoxilium group with the highest [K+] received Hemosol-B0 and the plasma [K+] following initiation of RRT was the same in both groups, suggesting that clinicians, appropriately, avoided Phoxilium in patients with high baseline [K+]. However, several patients with high baseline [HPO42-]pl were initiated on RRT with Phoxilium, reflecting the common observation that establishing a ‘default’ clinical pathway can inadvertently impede the application of clinical judgement. These data reinforce the view that a patient centred RRT strategy is preferable to a ‘one size fits all’ approach.
Our study further builds on previous work by standardizing the dialysis modality and dose, extending the study period to encompass the duration of RRT and measuring the important safety outcome of K+ supplementation.
LIMITATIONS
As a retrospective study the possibility of selection bias is acknowledged, in particular 9 patients received Hemosol-B0 during the ‘Phoxilium standard’ period. Clinicians may have favoured Hemosol-B0 in patients with high [K+] or [PO43-]. In a randomized study K+ containing CRRT fluid might be expected to promote hyperkalaemia precluding the safe undertaking of a randomized, blinded comparison.
Although obtained retrospectively, biochemical datasets resist ascertainment bias. This was a single-centre study albeit in a large metropolitan hospital with broad casemix.
FUTURE DIRECTIONS
Our outcomes are primarily biochemical, clinical outcomes associated with Hemosol-B0 vs. Phoxilium, or indeed RRT fluids in general, remain to be evaluated. We reviewed all RRT for AKI, the majority being short term. Studying a subgroup of patients requiring longer-term intensive care RRT might identify particular benefits of Phoxilium as hypophosphataemia becomes more problematic and other drivers of acidosis defervesce. Although units frequently designate a ‘standard’ RRT fluid, and indeed in the well-regarded RENAL study all participating centres agreed to use Hemosol-B0 for CVVHDF,5 with the availability of alternative RRT fluids such as Phoxilium trials evaluating patient centred RRT prescription are warranted.
CONCLUSION
In critically ill patients treated for AKI with continuous venovenous haemodiafiltration, Phoxilium was associated with hyperphosphataemia, hypocalcaemia and metabolic acidosis compared to Hemosol-B0. Phoxilium use reduced supplementary phosphate requirements; the presence of KCl in Phoxilium offers both advantages and disadvantages. These results inform clinicians of anticipated biochemical consequences of using these RRT fluids, and highlight the importance of patient-centred RRT fluid selection.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.






