INTRODUCTION
The natural history and management of genitourinary tumors in the pediatric age is different from that of the adults. Among the primary pediatric kidney tumors, the most frequent is the Wilm’s tumor, followed by the mesoblastic nephroma.1,2 Wilm’s tumor is the most common pediatric abdominal malignancy and the fifth most common childhood malignancy. Many histological variants of non-Wilms primary renal tumors are recognized, with prognosis and management varying by age and histology. Congenital mesoblastic nephroma is the primary diagnostic consideration for a renal mass in the neonate, although its incidence decreases with increasing age during infancy.3 Adrenal tumors occur rarely in childhood. Most antenatally detected suprarenal tumors are attributed to neurobalstoma. Neuroblastoma along with ganglioneuroblastoma and ganglioneuroma constitute a group of ganglion cell origin tumors that originate from primordial neural crest cells, which are the precursors of the sympathetic nervous system.
Ovarian malignancies in children may represent an array of unique problems for the clinician who is more accustomed to diagnosing and treating ovarian neoplasia in adults. Although ovarian malignancies in children are rare, their recognition and diagnosis are vital because they can be fulminant if treated inadequately. Tumors of germ cell origin (e.g., mature teratoma, malignant teratoma, endodermal sinus tumor (yolk sac tumor), embryonal carcinoma, dysgerminoma, primary choriocarcinoma) constitute approximately 70% of ovarian tumors in children.4 In addition to benign lesions, the differential diagnosis for a painless scrotal mass in a child includes leukemia, rhabdomyosarcoma, and a primary testicular tumor (germ or non-germ cell). Yolk sac tumors (endodermal sinus tumors) and teratomas are the most common primary pre-pubertal testicular tumors.5 All tumors of the bladder and urethra is rare in children; benign tumors are even more infrequent. Benign tumors in children described in case reports include polyps, papilloma, hemangioma etc. The most common carcinoma to involve the bladder is transitional cell carcinoma.
MATERIALS AND METHODS
This was a retrospective analysis of 28 cases of pediatric genitourinary tumors in surgical pathology department encountered over a period of 5 years. Surgical specimens and biopsy tissues received were fixed overnight in 10% buffered formalin and submitted for processing. Paraffin sections were cut at 4-6 microns thickness and routine Hematoxylin and Eosin (H & E) staining were performed. All cases were re-evaluated histologically on sections from routinely processed formalin fixed, paraffin embedded blocks. Special stains & Immunohistochemistry were studied wherever necessary. The clinical, radiological and therapeutic data were obtained from patients case paper records. Pattern of childhood malignancies were studied with a focus on tumor incidence, age and sex distribution, environmental and other etiological factors, demographic pattern, and histological type.
RESULTS
A total of 3149 pediatric surgical specimen obtained over a period of five years was analyzed. Of this, 28 were diagnosed with genitourinary tumors. Average incidence of genitourinary tumors pediatric tumors was 0.89%. The commonest individual tumor was wilm’s tumor followed by neuroblastoma. In the renal tumors only wilms tumors was seen, with classical triphasic tumors were more common. The mean age of presentation is 3 year with commonest age group of presentation in the age group 1-5 years (Table 1). Three of them had showed unfavorable histology. Among the gonadal germ cell tumors, we noted four mature teratoma, one immature teratoma, two yolk sac tumors of ovary & one yolk sac tumour in testis was seen. In the adrenal gland, adrenal medullary tumors were more common than adrenal cortex with neuroblastoma (4 of 10 cases) as common individual tumor (Tables 2 and 3). One case of squamous cell carcimoma of urinary bladder at the age of 10 years was documented and no any predisposing factor was elicited.
| Table 1. Age Distribution of Individual Tumor |
|
Tumor
|
0-1 yr
|
1-5 yrs
|
5-10 yrs
|
10-12 yrs
|
Total
|
| Mature teratoma |
|
1
|
2
|
1
|
4
|
| Immature teratoma |
|
|
|
1
|
1
|
| Yolk sac tumour |
|
1
|
|
2
|
3
|
| Wilms tumour |
1
|
8
|
|
|
9
|
| Bladder carcinoma |
|
|
1
|
|
1
|
| Adrenocortical adenoma |
|
|
1
|
|
1
|
| Adrenocortical carcinoma |
|
|
1
|
|
1
|
| Pheochromocytoma |
|
|
2
|
|
2
|
| Ganglioneuroma |
|
1
|
|
|
1
|
| Ganglioneuroblastoma |
|
1
|
|
|
1
|
| Neuroblastoma |
1
|
3
|
|
|
4
|
|
Total |
02
|
15
|
07
|
4
|
28
|
| Table 2. Gender Distribution of Individual Tumor |
|
Tumour
|
Male (M)
|
Female (F)
|
Total
|
M:F
|
| Wilms tumour |
7
|
2
|
9
|
3.5:1
|
| Bladder carcinoma |
1
|
|
1
|
1(M)
|
| Mature teratoma |
|
4
|
4
|
4(F)
|
| Immature teratoma |
|
1
|
1
|
1(F)
|
| Yolk sac tumour |
1
|
2
|
3
|
1:02
|
| Adrenocortical adenoma |
1
|
|
1
|
1(M)
|
| Adrenocortical carcinoma |
|
1
|
1
|
1(F)
|
| Pheochromocytoma |
1
|
1
|
2
|
1:01
|
| Ganglioneuroma |
|
1
|
1
|
1(F)
|
| Ganglio-neuroblastoma |
1
|
|
1
|
1(M)
|
| Neuroblastoma |
2
|
2
|
4
|
1:1
|
| Total |
14
|
14
|
28
|
1:1
|
| Table 3. Incidence of benign vs. Malignant Tumors |
|
Tumour
|
Total No. (n=28)
|
Percentage (%)
|
| Benign |
08
|
28.57%
|
| Malignant |
20
|
71.53%
|
DISCUSSION
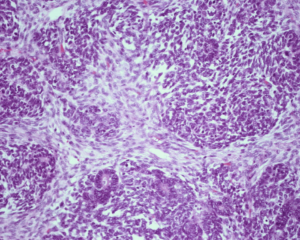
Childhood malignant tumors account for no more than 2% of all cancers.6 Tumors of the kidney, adrenal, ovary, testis and bladder represent a large part of the adult urologic practice, but are relatively infrequent in children. Among the primary pediatric kidney tumors, the most frequent is the wilm’s tumor (WT), followed by the mesoblastic nephroma. In the present study, wilm’s tumour with 9 cases was the largest group. All pediatric renal tumors were Wilms tumor i.e. 100% as compared to the 78.4% by Louisa Paul, et al.7 This difference may be due to small sample size in the present study. All the cases in this study presented with abdominal mass. Eight out of 09 cases were presented in an age group 1-5 years & 01 case was seen in an age group 0-1 year, which was comparable to the study done by Louisa Paul, et al.7 The median age of presentation was 3 years. Thus, the age distribution was consistent with other studies.7,8,9 Wilms tumor presenting in infancy when treated appropriately has a good outcome. Age 4 years at first diagnosis is clearly an adverse prognostic factor probably that due to adverse biologic features. There were seven cases in males & two in females giving a ratio of male to female ratio of 3.5:1. This was slightly on higher side as compared to study done by Louisa Paul, et al.7 Patel A. A., et al.10 in their study of 11 infantile Wilm’s tumour found the male to female ratio was 2.3:1. Husain A. N., et al.8 found Wilm’s tumour slightly more common in girls in whom it tends to present at an older age. Pathologically the mean tumour size in present study was 8 cm with a range of 4 cm to 15 cm (Figures 1 and 2). One of the poles of kidney was commonly affected. This was in accordance to the study done in the literature.7 Eight cases (88.89%) were classical (Triphasic) composed of epithelial, blastemal, and stromal elements and one case was monophasic type with predominantly epithelial component. Four other types of renal tumors can occur in childhood with sufficient frequencies are mesoblastic nephroma, clear cell sarcoma of the kidney, rhabdoid tumor, and renal cell carcinoma. Congenital mesoblastic nephroma is the most common renal tumor in infants.8 We have not observed these tumours in our present study.
Figure 1: Wilms Tumour Gross Pathology
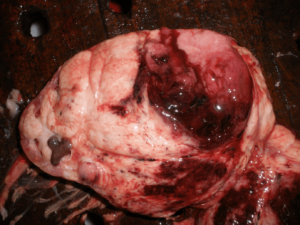
Figure 2: Wilms-tumor-triphasic Histology
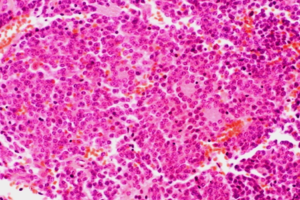
It has observed that, infants with neuroblastoma seemed to have better prognosis than older children even after minimal therapy.11 About 37% are diagnosed as infants, and 90% are younger than 5 years at diagnosis, with a median age at diagnosis of 19 months.11 In the present study, four of neuroblastoma cases were presented at the age of 11 months, 18 months, 3 years & 5 years with male & female ratio of 1:1. No overall sex predominance has been reported.12 All were presented with suprarenal or retroperitoneal mass & pain. Uncommon manifestations of NB related to unusual clinical behavior or to paraneoplastic syndromes were not seen in any case. Pathologically tumor cells forming the typical Homer-Wright rosettes arranged around the central fibrillary material without a central lumen or canal also seen (Figures 3 and 4). Mitotic activity was low in two of surgically resected neuroblastic tumors and absent in two of biopsy material. Calcification was noticed in all cases. The bone marrow biopsy record was not available in any case, which is also important in the monitoring of the disease activity.
Figure 3: Neuroblastoma Gross Pathology
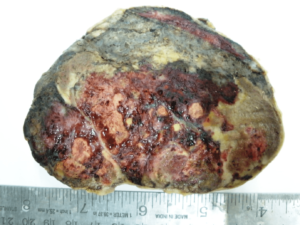
Figure 4: Neuroblastoma Histology
Some neuroblastomas have substantial internal morphologic variability about the degree of neuroblastic differentiation, ganglion cell maturation and Schwannian stroma. Differentiation in neuroblastic cells is recognized as neuropil formation and the acquisition of gangliocytic features. The phenomenon of differentiation has been codified by use of the term Ganglioneuroblastoma to denote intermediate differentiation and Ganglioneuroma to denote the fully mature neuroblastic neoplasm. One case of ganglioneuroblastoma at the age of 4 years in females was documented presenting as suprarenal mass.
Adrenocortical carcinoma are rare tumors that have a bimodal distribution, the first peak is in children less than five years and the second around the fifth decade.13 In our study, a single case of Adrenocortical carcinoma at an age of 7 years in a female child was observed and grossly have shown size of 15*12 cm, weight 650 gm, nodular mass with solid, cystic & necrotic areas. Microscopically the growth pattern was diffuse sheets of tumour cells with bright pink cytoplasm & broad mitotically active pleomorphic cells separated by broad fibrous bands and capsular invasion. Cagle, et al. specifically studied the adrenal cortical neoplasms in children; they found that only size (expressed as weight) was a reliable predictor of malignancy, with a weight greater than 500 g indicative of a carcinoma.8 The macroscopic features, presence or absence of necrosis and microscopic features, such as broad fibrous bands in the tumor, increased mitotic activity, capsular invasion, and a diffuse growth pattern helped to differentiate between adenoma & carcinoma.
Malignant germ cell tumors in the ovaries of very young children are exceedingly rare. In the present study one case was of immature teratoma, two cases of yolk sac tumour in ovary and one case of yolk sac tumour in the testis was documented. All three cases of the ovary presented at the age of 11 years & showed elevated levels of AFP. Yolk sac tumors are the second most common histological subtype (22%) of malignant ovarian germ cell tumor in children. Yolk sac tumors are the most common testicular germ cell tumor in childhood, representing in excess of 60% of cases and almost 50% of all testicular tumors in children.7 An asymptomatic scrotal mass in a child younger than 3 years of age are the common presentation. The histology and cytology of yolk sac tumors vary widely, often causing difficulty in diagnosis. The prototypic Schiller-Duvall bodies of endodermal sinus tumors are present in 50-75% of tumors.8 Yolk sac tumors are commonly associated with highly elevated serum AFP levels, which may be monitored clinically for recurrence and/or metastasis. In the present study, two of the yolk sac tumour was located at ovary & one at testis. All three had showed elevated levels of AFP. Both ovarian yolk sac tumors presented at the age of 11 years & one case of testicular yolk sac tumour at the age of 5 years. Grossly they have solid, yellow appearance. Microscopically polygonal tumour cells in reticular, trabecular & papillary pattern seen with Schiller-Duvall bodies. Microcystic change is seen in one ovarian yolk sac tumour.
All tumors of the bladder and urethra are rare in children. In the present study, one case of squamous cell carcimoma at the age of 10 years was documented and no any predisposing factor was eliciated. In contrast to adults, most pediatric bladder carcinomas are low grade, superficial, and have a good prognosis following transurethral resection. Rare cases of, leiomyosarcoma, and secondary involvement of leukemia, lymphoma, and Wilm’s tumor has been reported.8
To conclude, different types of genitourinary tumors are seen in the childhood. A high index of suspicion should be maintained with an aim of surgical treatment. Histological type is important for understanding etiology and progression of disease. The likelihood of a given type of tumor being present in a particular age or sex group or particular site may heighten the index of suspicion and ultimately influences etiology, biology, and natural history, relative incidence and distribution frequency, clinical presentation and manifestations, and response to therapy and outcome.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONSENT
The Patient has provided written permission for publication of the case details.









