INTRODUCTION
Acute kidney injury (AKI) is defined as a rapid loss of kidney function and oliguria, which is associated with adverse patient outcomes.1,2 AKI is frequently associated to care; it is estimated to occur in up to 15% of hospitalized patients and up to 60% of critically ill patients.3,4,5 Despite substantial advances in renal replacement therapy and health care delivery, morbidity and mortality rates associated with AKI have remained high. However, our current understanding of the epidemiology of AKI and its impact on morbidity, mortality, cost of medical care, and development of Chronic Kidney Disease (CKD) is based almost exclusively on studies of patients who developed AKI while hospitalized (HA-AKI). However, some patients develop AKI prior to hospitalization, termed community acquired AKI (CAAKI); the incidence of CA-AKI, the validity of the AKIN classification, and the impact of CA-AKI on patient outcomes are all not well studied. In this observational study, we compare clinical characteristics, etiologies, and outcomes of patients admitted to the hospital with community-acquired AKI in contrast to those who acquired AKI during their inpatient stay.
MATERIALS AND METHODS
Study Design
This is a prospective study, conducted during seven months from September 2012 to March 2013 in Hassan II University Hospital, Fez, Morocco.
Patients
We included all patients admitted to different departments of the university hospital and having acute kidney injury during the study period. Patient’s inclusion was done by nephrologist after his/her requestor an increased serum creatinine among those patients.
Comparing Groups Definitions
AKI was defined according to the acute kidney injury network (AKIN) classification6 (Table 1). Community-acquired acute kidney injury (CA-AKI) was defined as patients with sufficiently changed serum creatinine and urine output in order to meet AKIN criteria at the admission period (Table 1). Baseline serum Creatinine (sCr) values for patients with CA-AKI were determined through review of all sCr values taken from the patient (from the hospital or the community) during the preceding 12 months. Hospital-acquired acute kidney injury (HA-AKI) was defined as an increase in serum creatinine and/or oliguria, according to AKIN criteria, that occurred twenty-four hours or longer after hospitalization. Patients were identified as having HA-AKI if no AKI was apparent on admission to hospital, but AKI developed during their hospital stay. Baseline sCr for patients with HA-AKI was taken as sCr on admission and was confirmed to be representative of true baseline by review of results from 12 months earlier. When no baseline sCr was available, the percentage increase that defines AKI was calculated using the upper limit of normal laboratory reference range for sCr in men and women, respectively. Moreover, patients with unknown baseline values had sCr values charted after AKI resolution, which further enabled approximation of baseline sCr and confirmation of true AKI. This method of baseline sCr identification is recommended in the recent Kidney Disease Improving Global Outcomes (KDIGO) AKI guidelines.7
Table 1: Acute Kidney Injury Network criteria.
|
Stage
|
Serum creatinine criteria |
Urine output criteria |
| 1 |
Serum creatinine increase ≥26.5 μmol/l (≥0.3 mg/dl) OR increase to 1.5-2.0-fold from baseline
|
<0.5 ml/kg/h for 6 h |
|
2
|
Serum creatinine increase >2.0-3.0-fold from baseline
|
<0.5 ml/kg/h for 12 h |
|
3
|
Serum creatinine increase >3.0-fold from baseline OR serum creatinine ≥354 μmol/l (≥4.0 mg/ dl) with an acute increase of at least 44 μmol/l (0.5 mg/dl) OR need for RRT
|
<0.3 ml/kg/h for 24 h OR anuria for 12 h OR need for RRT |
Patients with preexisting chronic kidney disease (CKD) that sustained acute-on-chronic kidney injury were included. CKD was identified from blood tests indicating baseline eGFR< 60 ml/min per 1.73 m2 according to the Modification of Diet in Renal Disease (MDRD) equation.8 Recovery from AKI was defined as achievement of sCr no longer in keeping with the definition of AKI in comparison to baseline sCr values.
Data Collection
Data were collected by nephrologists practicing in the university hospital of Fez using a Case Report Form (CRF) that was designed earlier for the study. Clinical data collected included admitting specialty, demographics, medications, organ specific complications, and comorbid conditions. Creatinine values within 6 months prior to admission and at admission, at peak, at discharge were recorded. Admission to an Intensive Care Unit (ICU), requirement for dialysis, in-hospital mortality, length of stay, causes of death, in-hospital renal recovery and discharge disposition were recorded. A presumed cause of AKI was assigned based on clinical judgment after review of the medical record.
Statistical Analysis
Statistical analysis was carried out using SPSS software, version 20. A descriptive analysis was performed, Continuous data was presented as mean and Standard deviation (m±Sd) and categorical data as a percent and 95% Confident Interval (CI). At the univariate analysis, proportions were compared between groups using a Pearson chi-squared test. Continuous data were compared using t-test when comparisons were between two groups.
Ethical Considerations
An informed consent for participating in the study was obtained for all patients. No invasive investigation means was used. The authors declare no conflict of interest.
RESULTS
We included 210 patients having AKI, aged 57.2±19.2 years with a sex ratio (M/F) of 1.13.
The main reasons for hospitalization were infections, uro-nephrologic diseases and digestive symptoms in respectively 18%, 16.7%, and 14% of cases. Patients were admitted at emergency services in 66% of cases, at ICU in 15% of cases and at others medical departments in 16% of cases. Six percent of AKI episodes were mild (AKI stage 1); whereas most patients (70%) had sever renal insufficiency (AKI stage 3), and 24% stage 2. Length of hospital stay was a mean of 12.5±13.5 days. The global mortality rate among all patients study was 29%. Among the 210 patients with AKI, 157 were classified as CA-AKI (74.8%), while 53 cases were classified as HA-AKI (25.2%). There was no significant difference in age average between CA-AKI and HA-AKI. Preexisting CKD was observed in 15.7% of patients with AKI, with similar proportions across the CA-AKI and HA-AKI groups (16.5% versus 13.2%; p=NS). Comparison of prevalence of various comorbid conditions in patients with CA-AKI and HA-AKI revealed approximately equal proportions of such diagnoses as diabetes, hypertension, heart disease and cancer. Table 2 compares the patient characteristics of patients with CA-AKI and HA-AKI. The physiologic characteristics of AKI were divided into three categories; prerenal, intrarenal, and post renal. Intrarenal causes of AKI accounted for a greater proportion of HA-AKI (49% vs. 36%; p< 0.05), while prerenal causes were more common among patients with CA-AKI (48% vs. 43%; p=NS). Dehydration and volume depletion were significantly more prevalent in patients with CA-AKI (47.7% vs. 34%; p<0.04). Also the number of CA-AKI patients with glomerulonephritis as the cause of AKI was significantly higher compared with HA-AKI (10.1% vs. 1.8%; p<0.0001). The frequency of symptomatic congestive heart failure and obstructive uropathy was not significantly different between the two groups (Figure 1, Figure 2 and Table 3).
Figure 1: AC-AKI etiologies.
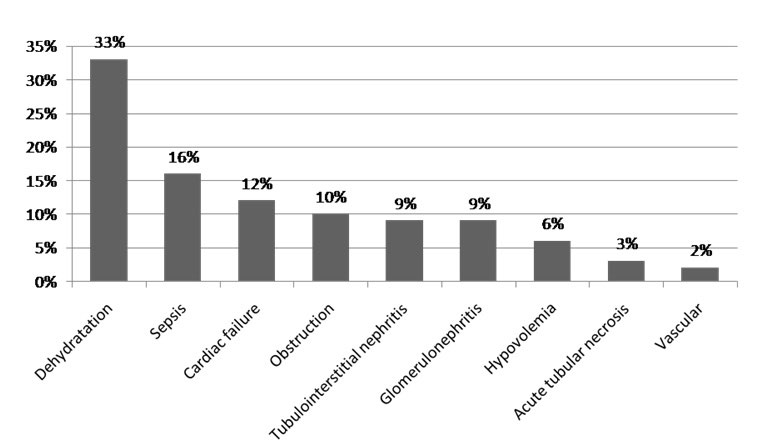
Figure 2: AH-AKI etiologies.
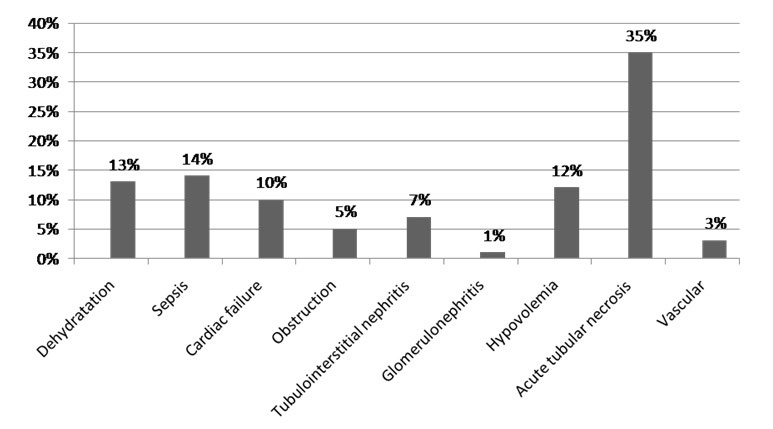
Table 2: Characteristics of patients with CA-AKI and HA-AKI.
|
IRA-AH (n=53)
|
IRA-AC (n=157) |
P
|
|
Mean age+/- SD (yr)
|
52.4+/-19 |
58.8+/-19 |
NS |
| Preexisting CKD |
7(13.2%) |
26(16.5%) |
NS
|
|
Diabetes
|
10(18.8%) |
30(19.1%) |
NS |
| Hyertension |
10(18.8%) |
29(18.4%) |
NS
|
|
Heart failure
|
6(11.3%) |
19(12.1%) |
NS |
| Cancer |
10(18.8%) |
24(15.2%) |
NS
|
Table 3: Etiology of AC- AKI and AH-AKI
|
Etiology
|
AC-AKI (n:157) |
AH-AKI (n:53) |
P |
| ATN |
4(3.8%) |
27(50%) |
<0.0001
|
|
Glomerulonephritis
|
16(10.1%) |
1(1.8%) |
0.042 |
| Volume depletion |
75(47.7%) |
19(34%) |
0.04
|
We have also investigated the data for acute mortality and short-term outcomes. Table 4 showed that the serum creatinine level at the admission was significantly higher in patients with CA-AKI compared to AH-AKI (62.5 mg/l vs. 42.6 mg/l p<0.01) Table 4 showed also no significantly differences between proportions of AKIN stages in the two groups compared. Short-term outcomes in patients with CA-AKI and HA-AKI are shown in Table 5. Having the same severity of AKI, the two groups had sustained a high rate of residual renal failure (77% AC-AKI versus 73% AH-AKI). Also there were no significant differences between the numbers of patients requiring renal replacement therapy in both groups (3.5% in the CA-AKI group and 1.8% in the HA-AKI group), and the length of hospital stay; the median stay in both patients with CA-AKI and HA-AKI was similar (12.5±14 days). However, mortality in hospital was significantly higher in the HA-AKI group compared to AC-AKI group (39.6% AH-AKI versus 25.4% AC-AKI; p<0.03).
Table 4: Severity of CA-AKI and HA-AKI.
|
AC-AKI (n:157)
|
AH-AKI (n:53) |
P |
| n |
% |
N |
%
|
|
AKIN stage1
|
11 |
(7%) |
2 |
(3.7%) |
NS |
| AKIN stage2 |
38 |
(24%) |
12 |
(22.6%)
|
|
AKIN stage3
|
108 |
(68.7) |
39 |
(73.5%) |
| Admission sCr values (mean±SD) mg/l |
62.5±57 |
42.6+/-33.8 |
<0.018
|
Table 5: Outcomes of community- versus hospital-acquired AKI.
|
AC-AKI (n:157)
|
AH-AKI (n:53) |
P |
| n |
% |
N |
%
|
|
Hemodialysis
|
35 |
(22.29%) |
11 |
(20.7%) |
NS |
| Residual renal failure |
121 |
(77%) |
39 |
(73%) |
NS
|
|
Mortality
|
40 |
(25.4%) |
21 |
(39.6%) |
<0.03 |
| Median length of hospital stay (days) |
12.59+/-13.4(1-71) |
12.4+/-14(1-81) |
NS
|
DISCUSSION
Incidence and associated mortality risks of AKI in critically ill patients are well documented.3,4,9,10 Increases in serum creatinine levels in non-critically ill hospitalized patients are also common and carry heightened mortality.1,2,11,12 This has been attributed to the older age and increased number of comorbid conditions present in hospitalized patients with AKI. In contrast, studies describing incidence, risk factors, and outcomes of patients who sustain AKI in the community are limited. The current study found that CA-AKI was more common than HAAKI, accounting for almost 80% of the patients with a diagnosis of AKI. That finding is consistent with two recent previous reports. Wonnacott, et al.13 identified 686 patients who sustained AKI in the community. They compared this cohort with 334 patients who sustained AKI during a hospital stay. The incidence of CA-AKI was found at 86.2% in this study. Also Schissler, et al.14 found higher incidence of CA-AKI at 80%. In two earlier studies, Obialo, et al.15 performed a retrospective study of 100 African Americans with AKI in which 80% of patients had CAAKI. Wang, et al.16 reported that 60% of 211 Chinese patients with AKI had CA-AKI. The absence of a reliable baseline serum creatinine was a significant limitation in both those studies. The availability for a baseline creatinine in the current study allowed us to accurately identify patients with CA-AKI, to define the prevalence of CKD in our cohort, and to accurately classify the severity of AKI.
This study highlights that risk factors for CA-AKI and HA-AKI are similar, with CA-AKI also being similar in patients with preexisting CKD, diabetes, heart disease, hypertension, and cancer. This highlights the clinical characteristics of people in the community who may benefit from more frequent blood tests in the event of an acute illness or medication change.
CKD was previously defined as a risk factor for AKI, and a 15.6% prevalence of CKD in the current study supports those observations.5,17 There was no difference in the prevalence of patients with CKD between CA-AKI and HA-AKI patients in our study. We deduce that CKD is also an important risk factor for CA-AKI. In a previous study, patients with CKD were reported to experience more severe AKI.17 However, the presence of CKD was not associated with increased severity of AKI in our study.
In agreement with other published reports,14 we also observed significant differences in the causes between patients with CA-AKI and HA-AKI. Volume depletion contributed to significantly more cases of CA-AKI, while ATN was more common in HA-AKI. This should not come as a surprise, since it is well known that patients with HA-ARF are more likely to have more severe illness, and the HA-ARF include frequently postoperative ARF cases.10
The need for acute dialysis in patients with AKI ranges from 36% to 86%,18,19 depending on the origin of the AKI and the hospital setting. A rate of 36% was reported in one community based study,19 while the rate was 46% to 86% in a hospital-based ICU study.18 In our study we observed no significant differences between the numbers of patients requiring renal replacement therapy in both groups (20.7% in HA-AKI and 22.3% in CAAKI).
Previous studies reported that RIFLE classification predicted increased length of stay, increased likelihood of discharge to rehabilitation facility, and increased mortality in patients with HA-AKI.1,12,20,21,22,23,24,25 In the current study the length of hospital stay was no different between patients with CA-AKI and HA-AKI and the degree of renal dysfunction cannot predict the length of hospital stay alone in the both groups because there was a similar distribution of AKIN class.
AKI is an important contributor to CKD. Previous studies have highlighted increased risks of de novo CKD following episodes of AKI with incomplete recovery.4,11,14,26,27 In the current study, patients with both CA-AKI and HA-AKI were found to have incomplete immediate recovery of renal function, based on discharge serum creatinine. We conclude that episodes of CA-AKI can also be a risk factor for the development or progression of CKD.
All notable adverse outcomes in AKI such as mortality occurred more frequently in HA-AKI. It has been previously noted that mortality in CA-AKI may be up to 20% lower than that of HA-AKI.18,19 According to some recent reports, the mortality rate in CA-AKI ranged from 15% to 26%,19 whereas the mortality rate in HA-AKI ranged from 25% to 70%.18 Also, the mortality rates observed in our study were consistent with these published reports. In this study, although AKI severity and comorbidity had a similar distribution between CA-AKI and HAAKI groups, the mortality rate was significantly higher in the HA-AKI group compared to AC-AKI.13,14 Documented predictors of mortality such as oliguria, sepsis, multiorgan failure, and ICU stay or mechanical ventilation occurred more frequently in patients with HA-AKI.24,25,26,27,28 In our study, we actually found the some finding, in fact, HA-AKI group had higher prevalence of mechanical ventilation (18.9% vs. 8.3% in CA-AKI group; p<0.05).
In the present study, having a long term following up of included patients would be relevant. It will allow us to determine renal long term outcome. This is a limitation for this study. However, we are confident about the results since the prospective design we used is very accurate.
CONCLUSION
This current report is one of few prospective study comparing AC-AKI and AH-AKI. Our data suggest that CA-AKI is a common cause of AKI that is as severe as that seen in HA-AKI. CA-AKI has a significant impact on length of stay, mortality, and the development and/or progression of CKD. Development of strategies to limit the risk of CA-AKI such as high risk factor subject screening may have a significant impact on healthcare costs and patient’s prognosis.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.







