INTRODUCTION
Hypomagnesemia has been described in several reports in adult patients on proton pump inhibitor (PPI) therapy. This potential side effect has not been studied in pediatric patients who usually require higher weight-based PPI dosing (up to 3.5 mg/kg/day) compared to adults.1
MATERIALS AND METHODS
Study Design
This was a retrospective cohort study assessing serum magnesium (Mg) levels in patients on PPI therapy followed by the pediatric gastroenterology service from September 1, 2003 to September 15, 2013. The institutional review board of University of Iowa approved the study. Patients were identified through an electronic search in our Electronic Medical Record (EMR) system using the following search parameters:
- Pediatric Gastroenterology encounter during the study period above, and
- PPI listed within the medication list, and
- Serum Mg level checked
For the purposes of this study, we defined the start of PPI therapy as the first time PPI therapy was documented in the pediatric gastroenterology outpatient or inpatient note or phone encounter. End of follow-up was defined as the time of the last documented pediatric gastroenterology note or phone encounter while using a PPI, patient death or end of study period. Chronic PPI therapy was defined as PPI use for more than 2 years. For patients with multiple Mg levels checked, only the lowest level was used from each 1 month period. Values were reported in mg per dL (mg/dL). The normal reference range used in our facility is 1.6-2.6 mg/dL. Cohort characteristics abstracted from the medical records included patient age and weight at time of starting PPI therapy, patient gender, indication of acid suppression and length of follow-up time by the pediatric gastroenterology service. Duration of PPI therapy was divided into the following categories: 0 to 1 month, 1 to 6 months, 6 to 12 months, 1 to 2 years, 2 to 4 years, 4 to 6 years, 6 to 8 years and >8 years.
Statistical Analysis
Continuous data were reported as means, medians, standard deviations and interquartile ranges. Categorical variables were described as percentages. Time trends of Mg levels while on PPI therapy were plotted with boxplot. Changes in Mg levels were assessed using Analysis of variance (ANOVA) for trends at time points. An unpaired Student’s t-test was used to compare Mg levels with and without chronic PPI use. The statistical significance was set at p≤0.05. Statistical analysis was performed with SAS 9 statistical software (SAS Institute, Cary, NC, USA) and GraphPad Prism 6 for Windows (Graphpad Software Inc., San Diego, CA, USA).
RESULTS
Cohort Characteristics
The electronic search using the criteria listed above resulted in 65 patients satisfying the search parameters. Of these, 34 patients were excluded from the analysis because of the following reasons: 28 patients did have not clear documentation of the time of initiation of PPI therapy, 4 patients had Mg levels checked before initiating PPI therapy and 2 patients had Mg levels checked after their last encounter with pediatric gastroenterology without documentation of continued PPI therapy. The remaining 31 patients had a total of 74 serum Mg levels and were included in the analysis. Cohort characteristics are shown in Table 1. The main indication for PPI therapy was Gastroesophageal Reflux Disease (GERD) in 69%. Some patients had more than one indication listed for using a PPI. Patients were followed by the pediatric gastroenterology service for a mean of 4.8 years. Fourteen patients qualified as being on chronic PPI therapy (>2 years).
| Table 1: Cohort Characteristics. |
| Characteristic (N=31 patients) |
Value |
| Age (years)
Mean (SD)
Median (IQR)
Weight (kg)
Mean (SD)
Median (IQR)
Gender (% male) |
7.77(±5.6)
7.05(10.1)
32.5(±21.8)
32.4(33.1)
68%
|
| Indication for PPI therapy (%)
GERD
Abdominal pain
Gastritis
History of tracheoesophageal fistula
Recurrent vomiting |
69%
19%
8%
4%
2%
|
| Length of follow-up time (days)
Mean (SD)
Median (IQR) |
1738(±1264)
1267(1899)
|
| SD: Standard Deviation, IQR: Interquartile ranges, GERD: Gastroesophageal reflux disease. |
Magnesium Levels
Two patients (6%) had Mg levels below the normal reference limit of 1.6 mg/dL on 9 separate measurements. In one patient, low levels were detected during the first 12 months of PPI therapy (on 7 separate measurements) in the inpatient setting. This was an 18 year-old male with acute leukemia (diagnosed one year prior) requiring matched sibling bone marrow transplant complicated by seizures, parasagittal sinus thrombosis, left frontal cortex intracranial hemorrhage, diarrhea and renal insufficiency. His chemotherapy regimen included methotrexate, vincristine, doxorubicin, prednisone and mercaptopurine. In the second patient, low levels were noted on 2 separate measurements in the inpatient setting between 4 to 8 years after start of PPI therapy. This was an 18 year-old male with Duchenne muscular dystrophy with voiding dysfunction, osteopenia, constipation and gastroesophageal reflux disease. Both patients had normal level documented on subsequent follow-up.
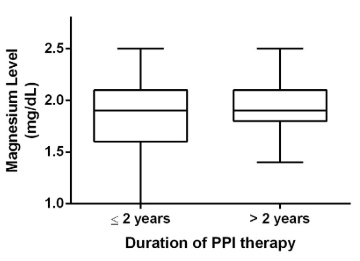
For the entire cohort, there was no statistically significant change in serum Mg level over time (Figure 1). There was no statistically significant difference between Mg levels checked while on short (<2 years) versus chronic PPI therapy (Figure 2). Categories of duration of PPI therapy and corresponding Mg levels are also presented in Table 2.
Figure 1: Boxplot analysis with magnesium levels from each time category. Each box extends
from the 25th to 75th percentiles. The line in the middle of each box represents the median magnesium level for that time category.
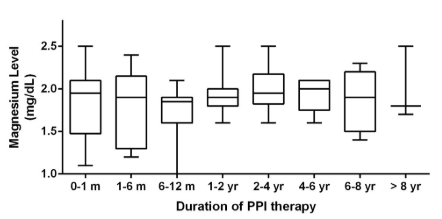
Figure 2: Boxplot analysis with magnesium levels from patients with and without chronic PPI therapy. Each box extends from the 25th to 75th percentiles. The line in the middle of each box represents the median magnesium level.
| Table 2: Trend of serum Magnesium levels over time. |
|
Duration of PPI therapy
|
Number of measurements |
Number of patients |
Mean Mg level (mg/dL) |
SD |
Median Mg level
(mg/dL) |
IQR |
| 0-1 month |
12 |
11 |
1.85 |
0.45 |
1.95 |
0.55
|
|
1-6 months
|
13 |
6 |
1.78 |
0.4 |
1.9 |
0.8 |
| 6-12 months |
14 |
5 |
1.75 |
0.32 |
1.85 |
0.3
|
|
1-2 years
|
12 |
6 |
1.93 |
0.23 |
1.9 |
0.2 |
| 2-4 years |
8 |
6 |
2 |
0.27 |
1.95 |
0.3
|
|
4-6 years
|
5 |
4 |
1.94 |
0.21 |
2 |
0.2 |
| 6-8 years |
7 |
5 |
1.87 |
0.37 |
1.9 |
0.7
|
|
>8 years
|
3 |
3 |
2 |
0.44 |
1.8 |
0.8
|
| SD: Standard Deviation, IQR: Interquartile ranges, Mg: Magnesium. |
DISCUSSION
Several reports have described the development of severe hypomagnesemia in association with PPI therapy in adult hospitalized and ambulatory patients.2,3,4,5,6Severe hypomagnesemia can present with tremor, changes in mental status, hallucinations, agitation, dizziness, tetany, seizures or cardiac arrhythmias.7,8 Discontinuation of PPI therapy typically results in rapid normalization of Mg levels within a few days with recurrence noted with resumption of PPI therapy. Oral or intravenous Mg replacement did not seem effective.8,9 The mechanism by which PPIs may be implicated in the development of hypomagnesemia is through reduced intestinal Mg absorption.10 It has been shown that Mg absorption can diminished by 1% in adult healthy volunteers taking 40 mg of a PPI.11 This may be related to interference with active transport channels as well as passive absorption.12
To our knowledge, there have been no published studies on hypomagnesemia in the pediatric population with PPI use. This study included 2 patients, both of adult age, with hypomagnesemia. The first patient had several other risk factors for hypomagnesemia including diarrhea, renal insufficiency and use of other drugs that may contribute to low Mg levels. Both patients recovered and were able to resume PPI therapy. One conclusion from this study is that hypomagnesemia is less common in the pediatric population than in adults possibly because of the lower prevalence of renal disease and use of diuretics that may be contributing to hypomagnesemia in the adult population. It is important to note that this study has several limitations including its retrospective nature, the small sample size and lack of long term consistent follow-up of serum Mg levels in on all patients. The study results could have been influenced by potential errors in EMR documentation of PPI use, lack of documentation of Mg levels checked at outside facilities and potential use of Mg containing supplements. Prospective pediatric studies assessing for changes in serum Mg levels during long term PPI therapy are needed to study the incidence and potential relationship better between PPI therapy and hypomagnesemia in children.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
CONSENT
The institutional review board of University of Iowa approved the study and need for consent was waived based on the retrospective nature of the study.







