Food is the basic necessity of life and food contaminated with toxic pesticides is associated with severe ill effects on the human health. Food legumes are an important part of the human diet, as these are good sources of proteins, carbohydrates and dietary fibres with satvik characteristics. On account of their high nutritive value they would play an important role in ensuring nutritional security especially for the developing countries.1 Keeping in view the fact that legumes are more susceptible to pest infestation, these are likely to be contaminated with certain chemical pesticides right from the crop growth to grain storage which may affect the food safety. Bengal gram or chickpea (Cicer arietinum) commonly called chana has been one of the widely consumed pulses in India. It is used in preparing a variety of snacks, sweets and condiments. Fresh green seeds are also consumed as green vegetable.2 Chickpea grains are usually stored for long periods in warehouses where various pesticides are intensively and successively applied many times resulting in their bioaccumulation. Many studies have shown that pesticide residues penetrate the grains and accumulate over time, thus indirectly exceeding the recommended doses. Therefore, studies in pesticide contaminated stored grain matrix under tropical conditions needs to be undertaken to study pesticide behavior and dissipation. In this regard, pesticide chlorpyrifos was selected for the present study as it is widely used pesticide for various applications including grain storage system.
In the initial phase of green revolution, chemical pesticides have contributed to the increase of yields in agriculture by successfully controlling pests and diseases.3 Inappropriate application of pesticides affects the whole ecosystem by entering the residues in food chain and polluting the soil, air, ground and surface water.4,5 As a result increasing incidences of cancer, chronic kidney diseases (CKDs), suppression of the immune system, etc. have been reported.4
Major pesticides used in crop production include organophosphates (such as malathion, chlorpyrifos), organochlorines (endosulfan, lindane, aldrin, dieldrin), synthetic pyrethroids (deltamethrin, cypermethrin, bifenthrin) and carbamates (carbaryl, bendiocarb) while in storage mainly pyrethroids (deltamethrin, cypermethrin, bioresmethrin) and organophosphates (malathion, chlorpyrifos) are employed.6,7,8,9 Chlorpyrifos is one of the world’s most widely used organophosphorus pesticides in agriculture. The use of chlorpyrifos has been restricted in US and some European countries but it is still in use in developing countries like India, where in the year 2000, it was the fourth highest consumed pesticide.10 Also during a survey in National Capital Region (NCR) in 2009, it was found that chlorpyrifos is the most consumed pesticides.11 Chlorpyrifos is bioactivated in the liver to chlorpyrifos-oxon, which then rapidly hydrolyzed to 3,5,6-trichloro-2-pyridinol (TCP). TCP has insignificant anticholinesterase (AchE) activity and is not regarded as toxicologically important, whereas chlorpyrifos-oxon is a potent cholinesterase inhibitor.12 Studies have indicated that the insecticide chlorpyrifos and its oxon metabolite inhibit acetylcholinesterase activity (AChE).
Some studies on pesticide residue dissipation in grains established that during storage the residues of pesticides were able to penetrate into the grains and accumulated with time.13,14,15 Stored grains being treated with chemical pesticides show presence of bound residues even after fairly long periods of storage contributing to dietary intake of pesticides.7
Food processing at domestic and industrial level would offer a suitable, simple and cost effective means to tackle the situation of pesticide contaminated food matrix. Processing techniques improves the nutritive value of legumes by reducing the anti-nutrients and enhancing bioavailability of micronutrients, the digestibility of protein and starch as well.16 Processing techniques like cooking, soaking and germination are also found to be effective in the dissipation of pesticide residues in food.17,18,19,20
The impact of food contaminants on metal bioavailability can be mediated by toxic damage of transport mechanisms, by metal-binding properties of the contaminant, or by its interaction with the homeostatically regulated distribution and excretion of essential metals.21 For instance, poisoning with mycotoxins like T-2 and deoxynivalenol (DON) reduces zinc absorption22 and the hepatic manganese content23 as was observed in animal experiments. Similarly the toxic pesticide residues present in food can interact with essential minerals, thereby affecting their bioavailability for the human body.24 So, it is necessary to investigate the impact of residues of commonly used pesticide on the mineral bioavailability also.
Literature reveals that though pesticide residue dissipation in selected food commodities through various processes has been well studied, little attention has been given to the persistence of pesticides in chickpea under storage conditions and their dissipation through domestic processing techniques. Further, in case of pesticide residue dissipation in food system, most of the research work overlooked the metabolites of pesticides which in some cases are even more toxic than the main pesticide. Also, an important aspect related to the bioavailability of micronutrients in the pesticide contaminated food matrix has not even been considered. Therefore, this research investigates the pesticide residues present in the grains under simulated storage (warehouse) conditions and their dissipation through processing.
MATERIALS AND METHODS
Reagents and Chemicals
Chlorpyrifos (20 EC) was purchased from Agrochemical dealer Dev Udyog, Nehru Place, New Delhi, India. The analytical standard of metabolite—TCP, PESTANAL (99.3% purity, 100 mg) was procured from Sigma-Aldrich and Chlorpyrifos-Oxon (94.0% purity, 50 mg) was of Dr. Ehrenstorfer GmbH make. All the reagents used in the experiments were procured from Qualigens Fine Chemicals, Mumbai, Maharashtra, India.
Sample Collection and Processing
The dissipation of chlorpyrifos in chickpea under simulated storage conditions was studied for which chlorpyrifos was applied according to the specifications given by Central Warehousing Corporation, 2007 according to which 20 EC chlorpyrifos diluted with water (1:19) is sprayed at a dose of 3 L/100 m2 . Gunny bags of 5 kg capacity were taken and filled with chickpea grains (2 kg) and placed on separate tables with plastic sheets spread over them. Then chlorpyrifos was sprayed at the recommended (6 g a.i/m2 ) and double recommended doses (12 g a.i/m2 ). The experiment was setup for 5 months (May-September 2009) in field laboratory (Micromodel Complex), IIT Delhi. The samples at various time intervals during storage were collected and analyzed for chlorpyrifos and its metabolites (oxon and TCP) by GC method.25 Three replications were taken for each treatment including control.
Recovery Studies
The levels selected for spiking with the pesticide standard after literature review were 1, 5 and 10 ppm. One kilogram of chickpea was finely ground in a domestic electric grinder out of which 50 g of ground chickpea sample was taken in conical flasks. The samples were spiked with 1.0 mL of 1, 5 and 10 ppm standard solutions to each conical flask. The samples were then kept at room temperature and homogenized by stirring with a glassrod until the solvent is evaporated.26
Sample Preparation
Twenty-five grams of sample was extracted with 200 mL acetone—water (8:2). The extracts were filtered through Buchner funnel using Whatman no.1 under suction. After rinsing twice with 25 mL of acetone the sample was concentrated in rotary vacuum evaporator over a water bath at 50 ºC to about 50 mL. The concentrated extract was taken in 500 mL separatory funnel and diluted with 250 mL of 5% aqueous sodium chloride and added hexane (100 mL thrice) for partitioning. Passed the combined layers of hexane containing pesticide residue through anhydrous sodium sulphate and concentrated to near dryness. The concentrate was taken in about 10 mL hexane for adsorption chromatography. In a glass column wet packed 5 g anhydrous sodium sulphate, 20 g of silica gel and 10 g anhydrous sodium sulphate bottom upward and pre-washed with 50 mL hexane. Just as the extract drained into the sodium sulphate, added 150 mL of 5% ethyl acetate in hexane and eluted chlorpyrifos. After concentrating as before, it was taken in a suitable volume of hexane for estimation by GC.27
Pesticide Treatment
Twenty emulsifiable concentrate (EC) of chlorpyrifos was diluted with water to form a stock solution of 1000 ppm which was further diluted to contain 10, 15 and 25 ppm solutions. One kilogram of chickpea thinly spread in a tray lined with aluminium foil was sprayed with the pesticide of above concentration. The grain was tumbled in a plastic vessel for half an hour and then a time-zero sample was taken for analysis.15
Determination of Bioavailability of Minerals
For determination of mineral bioavailability, chlorpyrifos was diluted to 1000 ppm and further diluted to contain 1, 5, 10, 15 and 25 ppm solutions which were applied to chickpea as mentioned in the “Pesticide treatment” section. Bioavailability of minerals from chickpea samples were determined by an in vitro method.28 All grain samples were finely ground in a stainless steel blender. The ground samples were subjected to simulated gastric digestion by incubation with pepsin (pH 2.0) at 37 °C for 2 h. Titratable acidity was measured in an aliquot of the gastric digest by adjusting the pH to 7.5 with 0.2 M sodium hydroxide in the presence of pancreatin—bile extract mixture (1 L of 0.1 M sodium bicarbonate containing 4 g pancreatin+25 g of bile extract). To simulate intestinal digestion, segments of dialysis tubing (molecular mass cut-off 10 kDa) containing 25-ml aliquots of sodium bicarbonate solution, being equivalent in moles to the sodium hydroxide needed to neutralize the gastric digest (titratable acidity) determined as above, were placed in Erlenmeyer flasks containing the gastric digest and incubated at 37 °C with shaking for 30 min or longer until the pH of the digest reached 5.0. Five milliliters of the pancreatin—bile extract mixture were then added and incubation was continued for 2 h or longer until the pH of the digest reached 7.0. At the end of the simulated gastrointestinal digestion, the minerals present in the dialysate were analyzed by atomic absorption spectrometry. The dialyzable portion of the total minerals present in the sample (expressed as percent) represented the bioaccessible minerals.24,29
Processing Techniques
The effect of simple domestic processing techniques like soaking and germination, ordinary cooking, pressure cooking and microwave cooking on chlorpyrifos and its metabolites was studied in chickpea seeds. Processed seeds were ground and analyzed for residues. The spiked unprocessed seeds had residue levels of 8.22, 11.95 and 22.41 ppm, respectively, for spiking at levels of 10, 15 and 25 ppm, respectively.
Soaking and germination soaked seeds were germinated for 24, 48 and 72 hours at 25 ºC in sterile petri-dishes lined with damp filter papers in incubator.
Ordinary cooking seeds were cooked in distilled water (1:5, w/v) on a hot plate for 120 min till they became tender.
Pressure cooking seeds were autoclaved at 15 lbs/inch2 for 20 min in beakers covered with aluminium foil containing distilled water (1:5, w/v).
Microwave cooking Seeds containing distilled water (1:5, w/v) were cooked in a domestic Samsung Bioceramic microwave oven (model CE283DN-850 W cooking power) for 15 min.30
After each type of processing, excess water was drained and the samples were dried in a hot air oven at 55 ºC for 24 h. The samples were then ground in a domestic electric grinder to pass through a 100 mesh sieve and then stored in resealable plastic bags at room temperature for further analysis.
Analysis of Chlorpyrifos and its Metabolites
Perkin Elmer Gas Chromatograph model Clarus-500 with NPD detector was used for analysis of samples. A sample of the reduced eluate (2.0 lL) was injected into the GC. Temperature programming for the above instrument was as follows: Temperature: 120 ºC for 3.00 min then 5 ºC/min to 270 ºC, hold for 10.00 min, detector: NPD at 300 ºC, injector temperature 250 ºC; carrier gas: helium at 1.5 mL/min. The retention times of TCP, chlorpyrifos and chlorpyrifos oxon were 5.29, 17.35 and 17.96 min, respectively.
Statistical Analysis
Mean and standard deviations were calculated from the results of the analysis performed. The data were subjected to analysis of variance (ANOVA). Means comparison was performed using Duncan’s Multiple Range Test (MRT). Significance was defined as p<0.05.
RESULTS
Effect of Simulated Storage Conditions on the Fate of Chlorpyrifos and its Metabolites
It is evident from the results that chlorpyrifos is able to penetrate the grains even in gunny bags over which a plastic sheet was spread. It is very peculiar to note that initially (0-20 days) the concentration of chlorpyrifos in the grains is less than that of its metabolite oxon (0.39 ppm and 1.37 ppm) which might be due to the rapid conversion of penetrating chlorpyrifos to oxon facilitated by high temperature and intense sunlight on roof of lab room during peak summer season of the study.31
With time, initially the accumulation of pesticide in chickpea grain increases and becomes maximum (4.61 ppm, recommended dose and 5.59 ppm, double recommended dose) after 30 days of storage (Figures 1 and 2). Even the concentration of its toxic metabolite oxon (2.66 ppm and 2.69 ppm at the two doses) is highest at this storage period. Further, the concentrations of chlorpyrifos and oxon decrease continuously during the remaining storage period. TCP which is not present initially appears after 30 days and then builds up in concentration and reaches maxima on 75th day and subsequently follows a continuous decline. It is probably as a result of conversion of oxon into TCP. The concentration of TCP becomes maximum after 75 days (3.56 and 4.35 ppm at two doses respectively).
The point that needs to be stressed here is that the maximum toxicity generated due to the metabolites is at 30th day (oxon concentration is maximum, toxicologically significant); though the calculated equivalent chlorpyrifos residues come out to be highest in chickpea grain samples after 75 days viz. 10.05 ppm and 11.55 ppm respectively. These two samples drawn at 75th day appear less toxic as compared to the grain samples taken in the initial storage phase (1 to 30 days) as the concentration of oxon is highest in this duration. As mentioned earlier that oxon is 3000 times more neurotoxic than the parent compound chlorpyrifos so the initial samples are highly unsuitable for consumption on account of very high toxic content. It is also clear that conversion of residues of metabolites into equivalent chlorpyrifos residues for ease of simplicity does not give correct picture of degree of hazards as it masks the very important fact of manifold toxicity of the metabolites.
Figure 1: Dissipation of chlorpyrifos and its metabolites in a chickpea matrix under simulated storage conditions (recommended dose, 6 g a.i m-2).
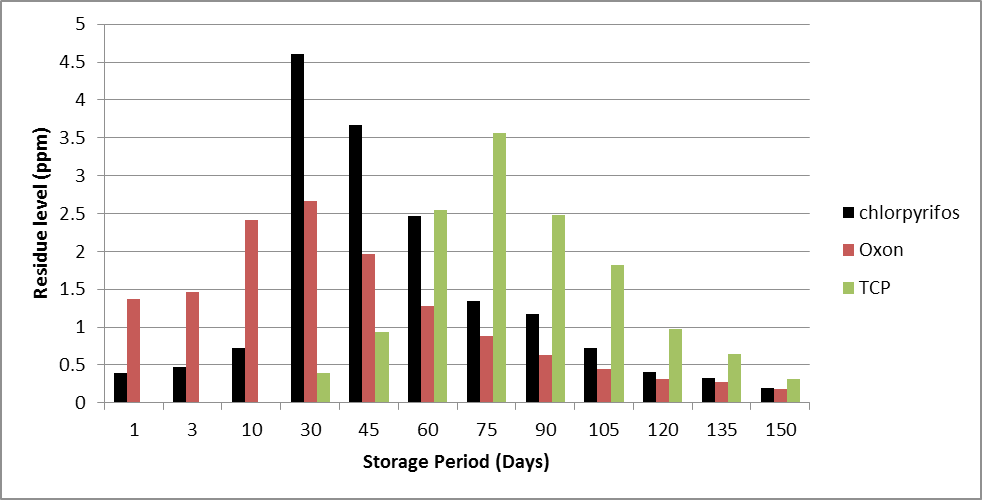
The dissipation pattern of chlorpyrifos was almost similar for both the treatments (i.e. recommended and double recommended dose), but slightly faster dissipation was noticed in the case of higher rate of application. This could be attributed to the fact that at higher dose of pesticide application, the quantity of chlorpyrifos available per unit area is more as compared to the lower dose of chlorpyrifos. Figures 1 and 2 give the dissipation pattern of chlorpyrifos and its metabolites under simulated storage condition.
Figure 2: Dissipation of chlorpyrifos and its metabolites in a chickpea matrix under simulated storage conditions (recommended dose, 12 g a.i m-2).
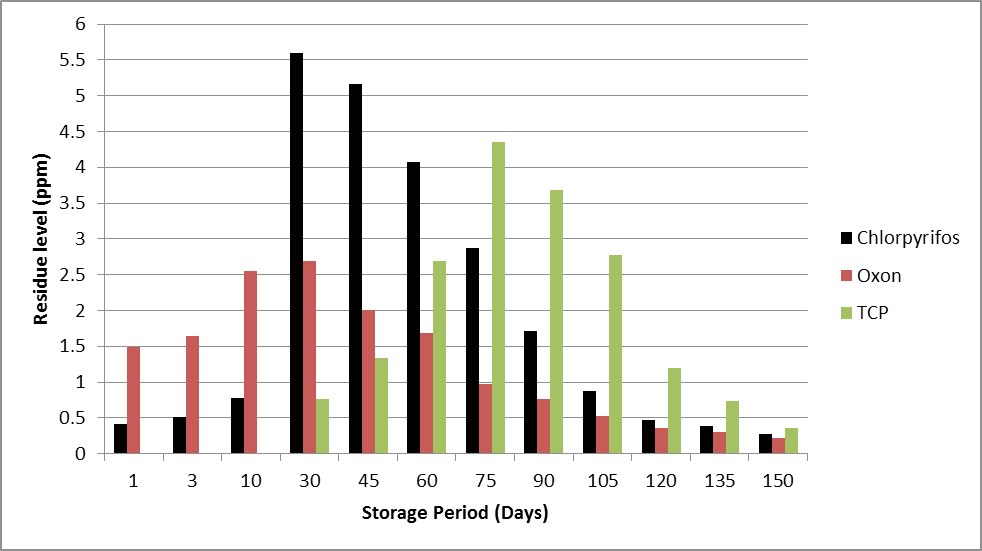
If the MRL value reported for chlorpyrifos in case of legumes (0.05 ppm) set by the European Union is extended to chickpea, the present data reveals that the residues of chlorpyrifos and its metabolites present in grains even after 5 months of storage are not at a safe level and may pose hazards if offered for consumption without decontamination.
Effect of Domestic Processing on Dissipation of Chlorpyrifos and its Metabolites
The chlorpyrifos treatment on chickpea grains was found to be persistent for protection from storage pests. Significant amount of residues (including metabolites) were observed even after 5 months of storage which indicates the concern regarding food safety. Hence, the effect of some simple, feasible domestic processing techniques on pesticide residues in chickpea was studied to see whether the grains could be made acceptable and safe for consumption. The processing methods include soaking coupled with germination, ordinary open cooking, pressure cooking and microwave cooking. Data are presented in Table 1.
Table 1: Effect of processing techniques on chlorpyrifos and its metabolites in chickpea.
Note: Values in parentheses of first column (Spiking level) denote actual level of initial residues. Results are means of triplicate±standard deviation. Different alphabets (a,b,c) in same row denote significant differences (p<0.05). ><0.05)
|
Spiking level
|
Chlorpyrifos and metabolites
|
Processing Techniques
|
|
Soaking & Germination
|
Ordinary cooking
|
Pressure cooking
|
Microwave cooking
|
|
10 ppm
(8.22 ppm)
|
Chlorpyrifos
|
0.15±0.03
|
0.19±0.05 |
0.25±0.06 |
0.28±0.02
|
|
Oxon
|
–
|
– |
– |
– |
| TCP |
– |
– |
– |
–
|
|
Equivalent Chlorpyrifos residues
|
0.15a
|
0.19 b |
0.25 c |
0.28 c |
15 ppm
(11.95 ppm) |
Chlorpyrifos |
0.18±0.04 |
0.26±0.07 |
0.15±0.07 |
0.18±0.05
|
|
Oxon
|
–
|
– |
0.16±0.05 |
0.44±0.02 |
| TCP |
– |
– |
0.31±0.10 |
0.34±0.06
|
|
Equivalent Chlorpyrifos residues
|
0.18 a
|
0.26 b |
0.86 c |
1.22d
|
|
25 ppm
(22.41 ppm)
|
Chlorpyrifos
|
0.29±0.09 |
0.78±0.06 |
0.47±0.09 |
0.20±0.01
|
|
Oxon
|
–
|
– |
0.19±0.06 |
1.05±0.06 |
| TCP |
– |
– |
0.36±0.05 |
0.45±0.04
|
|
Equivalent Chlorpyrifos residues
|
0.29a
|
0.78b |
1.30c |
2.05d
|
It is evident from Table 1 that germination (24 h) of chickpea seeds leads to almost complete dissipation of chlorpyrifos residues without the generation of its metabolites. Data shows that various cooking techniques used lead to high chlorpyrifos dissipation in chickpea but pressure cooking and microwave cooking were associated with generation of toxic metabolites oxon and TCP. Oxon is believed to be around 3000 times more neurotoxic than chlorpyrifos, the research findings reveal a very important concern regarding use of pressure cooker and microwave for cooking of legumes, as in ordinary open cooking, toxic metabolites are not generated at same concentration of chlorpyrifos treatment. At 15 ppm level of chlorpyrifos contamination, MW cooking generates almost 3 times more oxon than pressure cooking while in ordinary cooking oxon is not detected. With the increase in contamination to 25 ppm, the generation of oxon during MW becomes almost 5 times to that generated in pressure cooking.
Impact of Chlorpyrifos Residues on Micronutrient Bioavailability in Chickpea
In nutritional science, the bioavailability of an essential metal is determined by its metabolic utilization. For this purpose, the concept of “total utilization” defines the fraction of a nutrient that is ultimately used in metabolism after its digestion, absorption, and distribution.21
The data given in Table 2 clearly indicates that the bioavailability of micronutrients varied depending on the type of mineral and the concentration of the spiked pesticide. Bioavailability of iron and zinc did not change in pesticide contaminated chickpea (1-25 ppm). In case of manganese, the pattern was very different. An increase in bioavailability was noted with the increase in chlorpyrifos concentration up to 15 ppm but at higher spiked level (25 ppm) substantial decrease (54%) in bioavailability was observed. In case of copper there was slight but insignificant increase in bioavailability in chickpea grains spiked with chlorpyrifos concentrations ranging from 1 ppm to 10 ppm, however the bioavailability decreased significantly (26.66%) at the highest spiking level of 25 ppm of chlorpyrifos.
Table 2: Variation of mineral bioavailability (percent) with chlorpyrifos spiking.
Note: Results are means of triplicate±standard deviation. Different alphabets in same column denote significant differences (p<0.05).
|
Spiking Level
|
Fe
|
Zn
|
Mn
|
Cu
|
|
Untreated
|
0.88±0.21a |
1.56±0.16a |
0.65±0.07b |
0.15±0.04b
|
|
1 ppm
|
0.85±0.09a |
1.59±0.13a |
0.86±0.05c |
0.17±0.01b
|
|
5 ppm
|
0.91±0.03a |
1.49±0.04a |
0.79±0.04c |
0.16±0.02b
|
|
10 ppm
|
0.86±0.06a |
1.57±0.11a |
0.73±0.08bc |
0.16±0.05b
|
|
15 ppm
|
0.97±0.09a |
1.58±0.15a |
0.75±0.11bc |
0.15±0.01b
|
|
25 ppm
|
0.94±0.08a |
1.41±0.07a |
0.30±0.05a |
0.11±0.03a
|
DISCUSSION
Studies on chlorpyrifos residue under simulated storage conditions are scanty. Although, a similar study detected the presence of chlorpyrifos residues (0.2-0.8 ppm) in rice stored in jute bags for six months.32 A recent study in human patients revealed that formation of oxon occurred quite early (70 min) after ingestion of chlorpyrifos indicating a rapid conversion of the parent compound to its metabolite.33 In a similar study it was noted that after 6 months of deltamethrin treatment, 22-23% residues were present on the chickpea grains.8 In a study on malathion and its degradation products malaoxon, malathion α and malathion β-monocarboxylic acid were found in stored beans (4.10 ppm) and maize grains (2.79 ppm) treated with a mixture of pure radiolabelled malathion and 2% malathion dust even after 12 months of storage.7 High levels of malaoxon were observed in the wheat and bran samples during storage.15 It has been opined that the dissipation of pesticides after their application depends on various factors, including plant species, chemical formulation and application method,34 climatic conditions, physical phenomena (mainly volatilization) and chemical degradation in which sunlight plays a prominent role.35
Surprisingly, studies of pesticide dissipation during processing techniques on grain matrix are very scanty therefore mechanism involved cannot be elaborated. It is reported that, germination of broad bean seeds reduced pirimiphos methyl residues by 87%.36
In another study, steeping was found to be the most important processing step in the removal of fenitrothion and nuarimol residues (52%) followed by germination (25%) during the malting of barley.37 Recently, it was reported that cooking in a microwave oven causes a decrease in the residue levels of pesticides trifluralin, chlorpyrifos, decamethrin, cypermethrin and dichlorvos in rice and beans (pesticides spkied at levels of 1.0 ppm) when they were cooked in a microwave at 500 W and 800 W (power) for 15-45 min, respectively. After cooking, 92% to 99% of pesticides were eliminated.38 In a study on the effect of cooking on dichloro-diphenyl-trichloro-ethane (DDT) and its derivatives in green beans it was concluded that pressure cooking for 3 min at 15 psi resulted in a greater decrease in total amounts of DDT and its derivatives than microwave cooking for 6 min.39 A few studies for pesticide contaminated vegetables have been reported. For example, processing of spinach for 66 min at 122 o C reduced the pesticide residues and 100% dissipation of azinphos-methyl, 96% of malathion and 100% of methyl parathion was noted.40 Cooking of tomatoes at 100 o C for 30 min lead to 71.0-81.6% reduction in organophosphorous pesticides.17 In a recent study, it was found that boiling resulted in 100%, 92% and 75% organophosphate reduction respectively in brinjal, cauliflower and okra.41 It is inferred that processes involving heat treatment can increase volatilization, hydrolysis or other chemical degradation and thus reduce residue levels in cooked food.14,42
An important aspect of micronutrient availability in pesticide contaminated food has not been given attention, so data of the present study could not be corroborated. However, a few clinical studies report the impact of pesticide residues on mineral bioavailability. Pesticides did not affect the retention of zinc in the mucosa and small intestines of rats.43 Certain carbamates chelate with essential metal copper and reduce its availability in animals.44 Both lindane and linuron affect calcium metabolism.45 Dietary polychlorinated biphenyls (PCB), DDT and butylated hydroxyanisole (BHA) raised the level of copper in liver, kidney, or serum.43 2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin (TCDD) treatment resulted in an increase in the copper levels in the kidney and liver of rats.46 The incubation of hepatocytes for 60 min with 2, 4-D pesticide resulted in a significant increase in calcium to massive levels which was accompanied with the loss of cell viability.47
CONCLUSION
The dissipation pattern of chlorpyrifos and its metabolites in chickpea grains under simulated storage conditions revealed that chlorpyrifos and its metabolites were present in stored grains. Also, the residues exceeded the MRL values right from the beginning of the storage duration and even a 5 month period could not bring the residues below MRL. The chlorpyrifos residues were found 4 times (at the recommended dose) above the MRL highlighting the concern regarding safety of such stored grains for human consumption. The issue which further complicates the matter is the presence of metabolites viz. oxon (along with TCP) generated during grain storage which is even more toxic than the parent compound, chlorpyrifos. Here, it is important to note that existing MR values (by International Authorities) do not reflect any information about the harmful metabolites generated in grain matrix. Therefore, concerted efforts by considering toxic metabolites for evolving dietary guidelines for ensuring food safety have to be made. Fortunately, domestic processing techniques can thus reduce the residues of chlorpyrifos thus decontaminating the grains for human consumption. It was found that soaking and germination eliminated almost all the residues in stored chickpea while cooking processes also resulted in high chlorpyrifos dissipation but the build-up of toxic metabolite oxon especially during pressure and microwave cooking is a matter of great concern regarding food safety.
Further, in view of significant role of minerals in human metabolic activities, the effect of chlorpyrifos residues on the bioavailability of selected important micronutrients like iron, zinc, manganese and copper in chickpea was investigated. It was found that chlorpyrifos did not impact the bioavailability of Fe and Zn but significantly reduced the bioavailability of Cu (26%) and Mn (54%) at the highest spiking level of 25 ppm.
In fact, overall such studies reported in literature are scanty and scattered. Present systematic study focusing on metabolites of pesticide (chlorpyrifos) applied revealed very important findings related to food safety and nutritional security. Hence, fast cooking by microwave has to be recommended carefully and significance of traditional cooking system (through require more time) should be reinvestigated from the view point of food safety. Further, extensive and intensive investigation considering successive spray of various pesticides used in the warehouse for controlling storage pests needs to be carried out in the context of food safety.
ACKNOWLEDGEMENTS
Authors are grateful to Dr. K. Srinivasan for laboratory facilities at CFTRI, Mysore and Ms. Poonam Singhal (CRDT, IIT Delhi) for scientific discussion.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.