INTRODUCTION
Fasciolosis is a systemic parasitic disease of cattle caused by the liver flukes, Fasciola gigantica and Fasciola hepatica (trematodes of the genus Fasciola). Fascioliasis merits particular consideration due to its global distribution in livestock and the estimation of up to 17 million people infected worldwide. It is an important hepatic disease causing great enormous economic losses in ruminants and in livestock industries through animal mortality, growth retardation, sterility, condemnation of affected livers and expense due to control measures.1,2
Apart from its high veterinary importance, fasciolosis is a secondary zoonotic infection of a good number of human populations in every continent of the world. The increasing importance of human fascioliasis is also related to its pathogenicity and immunity. This disease is pronouncedly complicated, including difficulties in diagnosis, its great morbidity and immunological impact on children in long-term infection particularly in human fascioliasis endemic areas.3,4
Fascioliasis has the widest geographic spread of any vector-borne zoonotic disease occurring in more than 51 countries worldwide. In the Islamic Republic of Iran, Western Europe, Cuba, Egypt, and the Andean countries of South America about 91 million people are at a risk of getting infected.5
Since the liver is the main metabolic organ of the body, infection of the hepatocytes is an essential feature of certain parasitic infections. Due to the complexity of vascular and biliary system examination, there are a number of different laboratory tests, which are based on biochemical analysis of serum parameters. These tests usually include the determination of serum transaminases alanine transaminase, aspartate transaminase (ALT, AST), which are the most sensitive indicators of hepatocellular injury. Alkaline phosphatase (ALP), gamma-glutamyl tranferase (GGT), serum proteins and bilirubin are also used to evaluate the degree of cholestasis and synthetic capacity of the liver.6 In fascioliasis, the metabolic processes of the liver7 and kidney are gradually reduced.8
Chronic fascioliasis is accompanied by weight loss, weakening, pallor of mucous membranes, anemia, ventral edema, appetite changes, soft stool consistency or diarrhea, stomach hypotonia and decreased milk production.9 Clinical diagnosis and positive fecal egg counts along with elevated level of gamma-glutamyl transferase (GGT) confirm for the presence of chronic fascioliasis. The diagnosis of acute fascioliasis is implemented by the determination of serum hepatic enzyme activities which are released from the damaged hepatic cells.10
The aim of this study was to diagnose the functional capacity of the liver based on the activity of specific enzymes, proteins and glucose in serum and also to investigate the influence of mechanical and toxic effects of fascioliasis on blood picture and biliary tract of cattles.
MATERIALS AND METHODS
Ethical Approval
All study procedures were approved by and in accordance with the rules of animal use and care by the ethical committee of Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt.
Animals and Samples
This study was carried out between the months of January and August in the year 2016. A total of 150 animals were admitted to the Veterinary Clinic, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt because of altered appetite, weight loss and diarrhea in some cases. These animals were aged between 2 to 3 years and weighed between 350 to 450 kg. Fecal and blood samples were collected from 22 cattles with naturally acquired bovine fascioliasis and no other disease out of 150 cattles and 10 non-infected cattles. The selected cattles for study were confirmed to be free of other possible diseases.
Fecal samples were collected from the rectum for fecal egg counts. Fecal samples were examined by direct smear, floatation and sedimentation techniques for the presence of fluke eggs according to Urquhart et al.11 Two blood samples were taken from the jugular vein. The first sample was collected in evacuated Ethylenediaminetetraacetic acid (EDTA) tubes and used for hematological studies. The second was drawn into clean and dry serum separating tubes, left to clot and later centrifuged for 20 minutes at 2000 rpm for serum separation. Clear serum samples were carefully aspirated and stored at -20 °C to be used for biochemical analysis.
Hematological Studies
Total white blood cells (WBCs), red blood cells (RBCs), hemoglobin (Hb), packed cell volume (PCV), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) were calculated using an automated digital blood cell counter (Hospitex-Hemascreen 18, Fiorentino, Italy) using the veterinary software. Differential blood counts were determined using Giemsa-stained blood smears.
Biochemical Studies
Serum activities of aspartate aminotransferase (AST) and gamma glutamyl transferase (GGT) and serum concentrations of glucose, total protein (TP), albumin, blood urea nitrogen (BUN), and creatinine were determined according to standard procedures using commercial kits (Diamond, UK). Serum enzyme activities were determined at 37 °C. The concentration of globulins was calculated.
Statistical Analysis of Data
The data obtained was analyzed using SPSS version 16. The student t-test was used to analyze the significant differences between the hematological and biochemical parameters of the Fasciola-infected and the non-infected samples. Values of p<0.05 were considered significant.
RESULTS
Clinical Findings
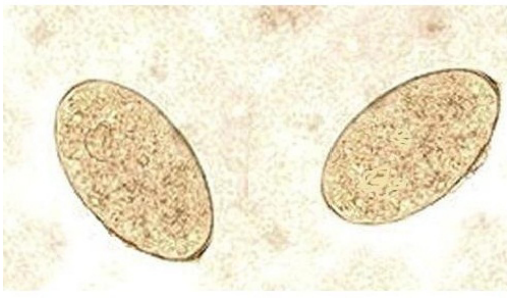
The infected animals showed prominent clinical signs of progressive weight loss and watery diarrhea. The mucous membranes were pale, with visible submandibular edema in some infected cases, and the rectal temperatures were all within normal range. Fecal examination revealed yellow brown thin walled, operculated eggs specific for fasciola species (Figure 1). Adult flukes of both species (Fasciola hepatica and Fasciola gigantica) are localized in the bile ducts of the liver or gall bladder. Fasciola gigantica measures 4 to 10 cm in length. Fasciola hepatica measures 2 cm to 3 cm in length and 1.3 cm in width, and has a cosmopolitan distribution. Fasciola hepatica is one of the largest flukes in the world. The adult worm has a characteristic leaf shape with the anterior end being broader than the posterior end and anterior cone and a ventral sucker at the base of the cone which allow it to attach to the lining of the biliary ducts. Each worm possesses ovaries and testes which are highly branched and allow for individual flukes to produce eggs independently.
Figure 1: Fasciola Eggs (X100).
Hematological Findings
The hematological results as shown in Table 1, indicated that RBCs counts, PCV, and Hb concentration were significantly (p<0.05) decreased in the infected cattle than in the uninfected control. No significant differences occurred between the erythrocytic indices (MCV, MCH & MCHC) of the infected and non-infected cattle indicating normocytic normochromic anemia. The results of leukogram of the infected and uninfected cattle are presented in Table 2. There was a significant (p<0.05) increase in the total leucocytes, neutrophils and eosinophils of the infected cattle with monocytopenia and lymphocytopenia in the infected cattle. No difference was observed in the values of basophils in both groups.
| Table 1. The Mean Values of the Erythrogram of the Uninfected and Fasciola-Infected Cattle (Mean±SE). |
|
Parameters
|
Uninfected group n=10 |
Infected group n=22 |
| RBCs (x106) |
7.40±0.33 |
5.00±0.11*
|
|
PCV (%)
|
38.15±0. 90 |
25.50±0.55* |
| Hb (g/dl) |
12.59±0.15 |
8.5±0.14*
|
|
MCV (fl)
|
51.55±0.94 |
51.00±0.43 |
| MCH (pg) |
17. 01±0.38 |
17.00±0.35
|
|
MCHC (%)
|
33.00±0.52 |
33.33±0.58
|
| Statistical significance between the infected group and infected group; *p<0.05 |
| Table 2: The Mean Values of the Total and Differential Leucocytic Counts of the Uninfected and Fasciola-Infected Cattle (Mean±SE). |
|
Parameters
|
Uninfected group n=10 |
Infected group n=22 |
| WBCs (x103) |
9.51±4. 67 |
14. 59±5.74
|
|
Neutrophils (x103)
|
3.45±1.02 |
7.85±1.27* |
| Eosinophils (x103) |
0.70±0.49 |
3.00±0.40*
|
|
Basophils (x103)
|
00 |
00 |
| Monocytes (x103) |
1.11±0.50 |
0.59±0.17*
|
|
Lymphocytes (x103)
|
4.25±0.86 |
3.15±0.61*
|
| Statistical significance between the infected group and infected group; *p<0.05 |
Biochemical Findings
The biochemical results (Table 3) revealed that the infected animals with fascioliasis had a significant (p<0.01) higher activities of serum AST and GGT with a significant (p<0.05) lower serum glucose, total proteins and albumin levels, as well as higher globulins levels than in the non-infected group. Moreover, the serum urea and creatinine levels were significantly (p<0.01) increased in the infected animals.
| Table 3: Biochemical Parameters of the Uninfected and Fasciola-Infected Cattle (Mean values±SE). |
|
Parameter
|
Uninfected group n=10 |
Infected group n=22 |
| AST (U/l) |
44.35±4.55 |
83.00±6.67**
|
|
GGT (U/l)
|
66.25±5.81 |
93.70±9.00** |
| Total protein (g/dl) |
7.55±1.24 |
5.97±0.62*
|
|
Albumin (g/dl)
|
3.53±1.09 |
1.10±0.76* |
| Globulins (g/dl) |
4.02±15.40 |
4.87±5.45*
|
|
Glucose (mg/dl)
|
55.07±1.17 |
40.16±3.27* |
| BUN (mg/dl) |
45.18±1.64 |
70.52±2.63**
|
|
Creatinine (mg/dl)
|
1.21±0.51 |
1.95±0.18**
|
| Statistical significance between the infected group and infected group; *p<0.05, **p<0.01 |
DISCUSSION
The progressive weight loss and pale mucous membranes in infected cattles may be attributed to anemia caused by Fascioliasis. Bottle jaw syndrome could be as a result of massive Fasciola infection causing liver destruction leading to cessation of protein synthesis.12,13 The distribution of the Fasciola species is limited to the tropics and has been recorded in South and Eastern Asia as well as in the Middle East, Eastern Europe and Africa.14 The clinical examination alone was not enough to diagnose the underlying pathogen; therefore, it should be used together with other laboratory diagnostic methods. The hematological results revealed normocytic normochromic anemia with decreased RBCs counts, PCV, and Hb concentration in the infected cattle may be attributed to chronic blood loss due to the blood-sucking activity of the adult flukes and leakage of blood from the bile duct to the intestine. The decrease in RBC counts can be due to decreased production or peripheral destruction of RBCs or blood loss from direct blood feeding by the flukes: Blood has been recovered from regurgitated caecal contents and from hemorrhage into the parenchyma, the bile ducts and the abdominal cavity as a result of activity of the fluke.9,15,16 The decrease in Hb concentrations may be due to deficiencies in iron implicated in fasciolosis and is a factor interfering with the synthesis of hemoglobin. The reduction in RBC counts, Hb and PCV in bovine fascioliasis may attributed to extensive loss of blood into bile duct due to the large amounts of flukes present in the liver or the acute loss of blood caused by the flukes.2,16 The severe anemia may be attributed to a chronic liver inflammation, which causes erythrogenesis depression.17,18,19 Similar results for RBC and PCV were observed in cattle,20 while the results for RBC and Hb were similar to those reported in cattle21 and in sheep.7,22,23 Moreover, the same observations in RBC, Hb and PCV were noticed in sheep with fascioliasis.10,24 This reduction was also found to be inversely proportional to worm load, which is in agreement with the previous findings,25 who reported that there is a good correlation between the level of infestation and RBC numbers, PCV, Hb content and plasma protein values.
Leucocytosis, neutrophilia and eosinophilia with monocytopenia and lymphocytopenia were reported in all infected cattles. These results were consistent with those obtained in sheep22 and bovine.2 The changes in the differential counts may be a means of body defense against Fasciola, obstructive effects or due to the toxin mediated lesion of the bone marrow.26 The neutrophilia and eosinophilia were also observed to be proportional to worm load. A positive correlation between eosinophil counts and the number of liver flukes recovered were previously observed.27 This may be due to inflammation and infection resulting from the activity of the flukes in the bile ducts.28 There are 3 main ways in which eosinophilia acts on the parasite. The mechanism of cytotoxicity cell-mediated antibody-dependent, in this case, the linking of the eosinophils to the target covered by various immunoglobulins; eosinophils may be linked with the targets covered by the C3b fragment of complement, exacerbating binding functions, causing damages to the parasite membrane; the interaction between immunoglobulin E (IgE) and eosinophils to obtain specific mediators, resulting in parasitic defense in two ways: local accumulation of neutrophils and other leukocytes, leukocyte activation and increasing their activity to damage parasites, and last would be action in the local tissue elements, intermediate local inflammation and producing unfavorable conditions for development of parasites. In addition, monocytopenia might be due to increased chemotaxis in response to the inflammatory process in the bile ducts.
With regards to the liver enzymes, the serum AST and GGT activities were significantly increased in the infected cattle comparatively with the uninfected. Liver enzymes activities are indicators of hepatic cell damage caused by fascioliasis.24,29 Fasciola hepatica causes the release of reactive oxygen species causing cell wall damage and hepatic tissue necrosis.30 These changes influence the biochemical parameters in the serum, and determination of specific liver enzymes functions as a valuable tool for diagnosis of hepato-biliary diseases. Increased values of serum AST could be related to the hepatocellular necrosis and degenerative changes produced by migration of immature flukes through the liver parenchyma. Moreover, the cellular changes from parasitism increase the permeability of the hepatic cells and in turn result in the release of the enzymes into the serum. Elevation in serum GGT levels was an indicator of chronic changes, cholestasis and epithelial damage in bile ducts caused by presence of adult flukes in biliary tract. These results agree with studies showing that a GGT increase provides sensitive indications of liver injury. GGT is the best marker, while AST is less sensitive.24 Moreover, our findings were confirmed with those obtained previously in cattle and bovine,31 cattle19 and ruminants.32 They reported degenerative changes in the hepatocyte and biliary cirrhosis in the liver histopathology of cows and cattle infected with Fasciola.
Hypoproteinaemia, hypoalbuminaemia and hyperglobulinaemia produced in this study were due to fascioliasis. This observation is in agreement with other studies in the cattle,33 in fallow deer,29 in sheep10 and in bovine.8 The hypoproteinaemia is due to severe infection of the liver produced destruction of liver parenchyma resulted in drastic alteration in protein values. The hypoalbuminaemia may be due to reduced albumin synthesis caused by liver damage. This produces cholangitis, biliary obstruction, destruction and fibrosis of hepatic tissue and anaemia.28 The detected hyperglobulinaemia could be as a result of immune response to infection10 and due to the increase in α and β globulin production.34 The consequences of liver damage resulting from the migrating flukes compromises liver function which is reflected in changes of plasma proteins (albumin, globulins) concentration.32
The observed hypoglycemia may be attributed to the disturbance of glucogeneogenesis which results from hepatic disorders35; elevation of the ketone bodies during gastroenteritis which results in blood glucose depression34 and depression of the hepatic glucogenic pathways and decrease in voluntary feed intake by the infected animals. Liver parenchymal damages inflicted by higher worm load could possibly be the cause of hypoglycemia.8
Elevations of serum urea and creatinine levels were due to Fasciola infection in cattle. These results may be due to renal problems. In agreement with others36 who reported that glomerulopathy is associated with fascioliasis and buffaloes are suitable as a naturally existing experimental model of renal injury by circulating immune complexes.
CONCLUSION
It could be concluded that measuring the hematological and biochemical parameters could be useful in early diagnosis and prognosis of cattle fascioliasis. Additionally, changes in the levels of hepatic enzymes (AST and GGT) released into the blood as a result of liver tissue damage are used to monitor the progress of the infection and as a sensitive diagnostic aid in field infection.
ACKNOWLEDGMENTS
Special thanks to Clinical Pathology and Parasitology Departments, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt that supported us during conduction of this research. This study was funded by authors and no outside fund was received.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interests.