INTRODUCTION
The skin is the first barrier of our body with important protective functions. Its protective role is dependent on the sebum, a complex mixture of lipids, triglycerides, wax esters, cholesterol esters, sterols, squalene and free fatty acids, all essential components for the formation of the hydro-lipid film which coats the skin and the scalp. The function of the hydrolipidic film is to lubricate and moisturize the skin surface, allowing the skin to maintain an appropriate level of hydration and protecting it against microbial invasions.
Sebum is produced by small specialized organs, the sebaceous glands, which are located over the entire body, with the exception of the hand palms, feet soles and some genital areas: they are more abundant and productive on the face and scalp, and usually associated with hair follicles. Although the sebum has got an important protective role for the skin, its overproduction by the sebaceous glands may lead to aesthetic problems and even more serious skin disfunctions.
Seborrhea is one of the most common cosmetic problems affecting both men and women, typically highest in the age range of 15-35 years and declining throughout the adult age range,1 and affecting more Asian than Caucasian people. Seborrhea gives an oily, shiny and greasy aspect to the skin and it is frequently accompanied by large pores. Many individuals feel embarrassed and annoyed with the appearance of their oily skin, often associated with a feeling of dirtiness.
Sebum production is influenced by several factors, among which the most important is the level of the androgen hormone testosterone: indeed the massive activation of the sebaceous glands occurs during puberty, when the production of testosterone is more intense. The testosterone concentration influences the production of sebum as it is converted, at level of sebaceous gland, into dihydro-testosterone by the enzyme 5-α Reductase. There are two isoenzymes of 5-α Reductase, the 5-α Reductases 1 (5αR1) and the 5-α Reductases 2 (5αR2), both of them have the capacity to convert testosterone into dihydrotestosterone, but they have different tissue distribution and functions.2 The 5αR1 is mainly expressed by sebaceous cells, whereby 5αR2 is preferentially produced in the hair follicles and prostate; moreover, the activity of 5αR1 is significantly higher in sebaceous glands from face and scalp compared to non-acne prone areas.3 Thus, a down regulation of 5αR1 in the skin represents one of the solutions to contrast excessive sebum production, and some other strictly related diseases, such as seborrheic dermatitis and acne.
In seborrheic skins the overproduction of sebum is usually associated to a massive bacterial proliferation, more commonly the species of Staphylococcus and Propionibacterium acnes, because the sebum contains nutrients and thus represents a substrate for bacteria growth.4,5 The β-defensins, small cationic peptides, are the major components of innate immunity, a non-specific immune defense response against microorganism attack and hyper-proliferation. They have a strong antimicrobial, antiviral and antifungal activity,6 since their particular cationic nature allows to destroy the microbial membrane, negatively charged, by binding it, and leave intact human cell membranes that have a neutral charge. Thus defensins represent the initial defense response against microbial invasion. To date, three human β-defensins (hBD) have been shown to play a role in the defense of the skin, namely human β-defensin1, 2 and 3 (hBD1, hBD2, hBD3). They are expressed in all human epithelial tissues, either constitutively or up regulated in response to microbial or pro-inflammatory stimuli.7 In patients with acne the expression of the hBD2 is increased in the sebaceous glands,8 where it is likely to exert an important role in protecting from microbial invasion.
Among the unicellular organisms, the red microalga Galdieria sulphuraria has attracted the attention of applicative research, because it has well adapted to the extreme environment of the volcanic calderas9: it is able to withstand high temperatures and grow in high-sulfur growth media, at pH-1. Its presence on the walls behind the Bocca Grande of volcanic crater Solfatara at Pozzuoli, near Naples, has been documented for several years.10 Recent studies have shown that this microalga is capable of synthesizing a wide range of antioxidant compounds, including the sulfur containing glutathione, making it an excellent candidate for skin care applications.11 Indeed, the use of sulfur in dermatology has demonstrated efficacy in the treatment of many dermatological disorders including acne vulgaris, rosacea, seborrheic dermatitis.12,13 Moreover, for its high content of vitamins, essential fatty acids, proteins, carotenoids and phycocyanins, Galdieria has even been proposed as dietary supplement and food ingredient.14
On the basis of these properties, we investigated the potential benefits of a hydrosoluble extract obtained from Galdieria sulphuraria on the skin, in particular as cosmetic ingredient aimed at balancing sebum production. We demonstrated that G. sulphuraria extract was able to reduce the 5αR1 expression in skin cells, to reinforce the skin’s natural defense system by up regulating hBD2 expression and to promote the skin integrity accelerating the wound healing process. A clinical study performed on human volunteers confirmed the efficacy of G. sulphuraria extract as potential active ingredient for skin care applications, particularly directed to patients with oily and seborrheic skin.
MATERIAL AND METHODS
Galdieria sulphuraria cultivation and extract preparation
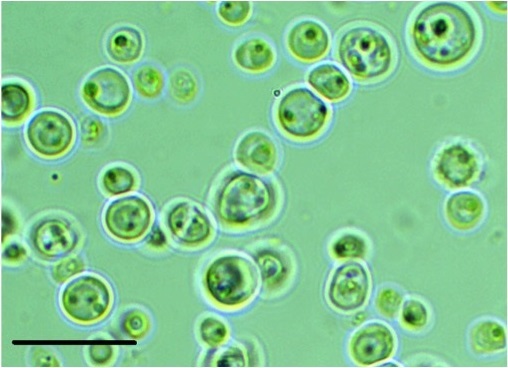
G. sulphuraria (strain n. 017) was obtained from ACUF (Algal Collection of the University of Naples Federico II, Italy), originally collected from a thermal spring site in Pozzuoli, Napoli (Italy). Stock cultures of axenic G. sulphuraria strain were maintained routinely on both liquid and agar slants of Allen medium15 in heterotrophic conditions, being the glucose (30 g L-1) the main source of organic carbon (Figure 1). The biomass production was carried out in batches, and the culture flasks were incubated at a temperature of 26±2 ° C with continuous air insufflation. After 2 weeks of growth, the cultures were harvested by a continuous centrifuge (2,500 g); the collected biomass was rinsed with 10 L of distilled water and frozen in sterilized containers. For the preparation of G. sulphuraria extracts, the frozen G. sulphuraria biomass was suspended in two volumes of phosphate-buffered saline (PBS) pH 7.4, and homogenized in an industrial homogenizer. The resulting lysate was centrifuged at 4,000 rpm to precipitate the particulate fraction and isolate the soluble components. The supernatant was then collected, adjusted to pH 6.5 using NaOH, and lyophilized until obtaining a dry powder, that represented the Galdieria sulphuraria Hydrosoluble Extract (GsHE). The powder was then dissolved in water or cell culture medium at the required concentrations for the different analyses. Three independently grown batches were tested in the different experiments.
Figure 1: Microscopic picture of the microalga Galdieria sulphuraria taken by an Inverted Microscope Motic BA. Magnification=1000 X; the black bar corresponds to 20 μm.
Cell Vitality Assay (MTT Assay)
1.3×104 cells (keratinocytes, HaCat) were seeded in each well of a 96-well plate, grown for 8 h and treated for 48 h with different concentrations of GsHE. After treatments, cells were washed with PBS and incubated with 100 µl per well of “reaction buffer” containing: 10 mM Hepes, 1.3 mM CaCl2, 1 mM MgSO4, 5 mM glucose and 0.5 mg ml-1 of colorimetric substrate MTT [3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazoliumbromide] in PBS buffer at pH 7.4, according to the method described by Mosmann et al.16 After 3 h at 37 ° C and 5% CO2, cells were solubilized by the addition of 100 microliters of solubilization solution (10% TritonX-100, 0.1 N HCl in isopropanol), and the plate incubated for 4 h at room temperature. The number of healthy cells was directly proportional to the level of the formazan product created. The developed color was then quantified at 595 nm by the multi well-plate reader Victor3 (Perkin Elmer, Waltham, MA, USA).
5 α-Reductase type 1 and human β-Defensin 2 gene expression analysis
For the gene expression analyses of the 5-α Reductase type 1 (5αR1) and the human β-Defensin 2 (hBD2), Human Dermal Fibroblasts (HDF) and keratinocytes (HaCat) were used respectively. The cells were incubated with GsHE for 6 or 8 h, and then processed for RNA extraction using a RNA Purification Kit according to the manufacturer’s instructions (GenElute Mammalian Total RNA Miniprep Kit, Sigma-Aldrich). Reverse transcription polymerase chain reaction (RT-PCR) was performed using gene-specific primers and an oligo-nucleotide internal standard Kit (Quantum RNATM 18S Ambion, Austin, Texas, USA). The PCR products obtained were visualized and quantified with an Imaging system (Geliance 200, Perkin Elmer, Waltham, MA, USA). The amplification band corresponding to the analyzed gene was normalized to the amplification band corresponding to the internal standard 18S. The values obtained were finally converted into percentage values by considering as 100% the measure of the samples of untreated cells.
Scratch Assay
For the scratch assay, HDF were seeded in 6-well plates. 12 h later, same areas of each well were displaced by scratching a line through the cell layer by a pipet tip and floating cells were removed by PBS washing. Culture medium containing 0.5% FBS with or without GsHE was added and the cells incubated for 7 hours. Mitomycin C (10 µg µl-1) was always included in the medium to prevent cell proliferation and valuate the cell migration capacity. To estimate the wound healing capacity of the cells, the unclosed cell-free area at time 0 and the one at 7 hours after the treatments were compared for each condition by using the software Image J.
Clinical Test
The study was performed on 20 male and female volunteers, aged from 18 to 50, who had an acne-prone face skin, although not suffering from any disease to the skin areas to treat. Each subject applied a cream containing 0.002% of GsHE, twice a day for a period of 30 days on the right side of the face massaging until absorption; the left side was treated with a placebo and the forehead remained untreated for the entire duration of the study. The amount of skin sebum excretion was measured on the lateral creases of the nose and on the forehead, before the treatments (T0), and after 15 days (T15) and 30 days (T30) of a bi-daily application of the product, by using the Sebumeter® SM815 (Courage-Khazaka GmbH, Germany), a digital probe for instrumental measure of the cutaneous sebum amount.17 The study was carried out under standard environmental conditions for each reading time, maintaining constant room temperature and monitoring humidity by a Thermo-hygrometer (Taylor Precision, NM).
RESULTS AND DISCUSSION
Calculation of the usage doses for GsHE To evaluate the potential cell toxicity and determine the correct doses of GsHE to use in the bioassays, we performed a cytotoxicity test (MTT assay) by testing scalar concentrations of GsHE, ranging from 0.5% to 0.0006%, on HaCaT keratinocytes. After 48 h, the vitality rate was determined. The IC50 calculated was 0.198 %, and the highest non-toxic dose (which gave no mortality) was 0.01% (data not shown). Thus, the concentrations of GsHE chosen for the studies on skin cells were 0.002 and 0.01 %.
GsHE on 5αR1 Expression
In order to study whether G. sulphuraria had the capacity to affect the activity of 5αR1, the main enzyme regulating sebum production in the skin, we used GsHE at the 2 established concentrations to treat HDF for 6 h. Fibroblasts express 5αR1 in normal conditions and therefore are an appropriate model to investigate 5αR1 activity and regulation.18 After treatment, the cells were collected, RNA extracted and the expression of 5αR1 gene analyzed by RT-PCR. As shown in Figure 2, the treatments with both the concentrations of GsHE reduced the expression of 5αR1 (-45% for 0.01% and -35% for 0.002%), suggesting a potential role of the extract in reducing the sebum production in the skin by acting through an inhibition of 5αR1 expression.
Figure 2: RT-PCR analysis of gene expression in fibroblasts treated with GsHE at the indicated concentrations. After RNA extraction, the expression level of the 5αR1 gene was analyzed by RT-PCR.
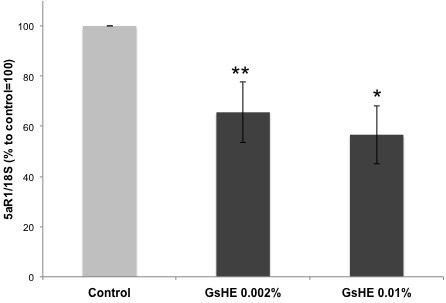
The values obtained from the PCR band quantification were normalized to the standard amplification bands of 18S, reported in the column graph and expressed as percentages to the control, arbitrarily set as 100%. The error bars represent standard deviations, and the asterisks indicate statistically significant measures (*p<0.01; **p<0.001).
GsHE on hBD2 Expression
As explained in the introduction, an overproduction of sebum can be often associated to a hyper proliferation of microbes in the skin, leading to skin injuries and to the triggering of inflammatory processes. Therefore, β-defensins represent an efficient defense mechanism developed by the skin cells to fight microbe proliferation. In order to test the ability of GsHE to induce the skin’s natural defense system, gene expression analysis of hBD2 was performed in human keratinocytes treated with the two different concentrations of GsHE. The keratinocytes are the cells mostly exposed to microbial attacks, thus more keen to respond by activating a rapid production of inducible β-defensins. As shown in Figure 3, both the doses of GsHE increased hBD2 expression, but the lowest concentration of 0.002% was even better than the higher one to induce hBD-2 (170 % increase versus 60%), suggesting a good potential stimulatory effect on the innate skin defense system. Three independent extract preparations gave all the same result, indicating that this inverted dose response relationship was reproducible. The higher efficacy of the lower dose compared to higher one could be explained by considering that the extracts are mixtures of several types of molecules that may act synergically or counteract each other whether negative regulators become predominant. In this case, the choice of the right concentration of the extract would be of primary importance to produce a final positive effect on the cells both in vitro and in vivo.
Figure 3: RT-PCR analysis of gene expression in keratinocytes treated with GsHE at the indicated concentrations. After RNA extraction, the expression level of the BD2 gene was analyzed by RT-PCR.
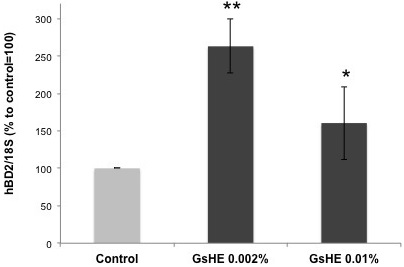
The values obtained from the PCR band quantification were normalized to the standard amplification bands of 18S, reported in the column graph and expressed as percentages to the control, arbitrarily set as 100%. The error bars represent standard deviations, and the asterisks indicate statistically significant measures (*p<0.01; **p<0.001).
GsHE In Wound Repair
Wound healing is a natural restorative response to tissue injury, regulated by several proteins and secreted signals, which orchestrate the interactions among all the cells forming the skin. Recent studies have shown that hBD2, besides their roles as antimicrobial peptides and chemo attractants, play an active role in several growth-dependent processes as well, like wound healing and cell migration or proliferation.19–21 Furthermore, the increased expression of hBD2 can positively affect the wound healing process, which could be an additional advantage for the treatment of skin microlesions and scars linked to seborrhea.
To test the ability of GsHE to promote the wound healing process, a scratch assay was performed on fibroblasts treated with the extract. In a typical scratch assay, a “wound gap” in a cell monolayer is created by scratching, and the “healing” of this gap by cell migration towards the center of the gap is monitored and quantified. As showed in Figure 4 (A,B), GsHE increased the capacity of the cells to repair the wound: it accelerated the cell migration and the healing process by 17% at the concentration of 0.002%, compared to the untreated control, similarly to the Transforming Growth Factor-β (TGF-β), known for its ability to accelerate the wound repair.22 Also in this case, there was a slight difference between the two doses, confirming that the lower dose was the one giving a better positive effect on the cells.
Figure 4: Scratch assay. Confluent fibroblast mono-layer cultures were scratched by a pipet tip and then treated with GsHE or the growth factor TGFβ.
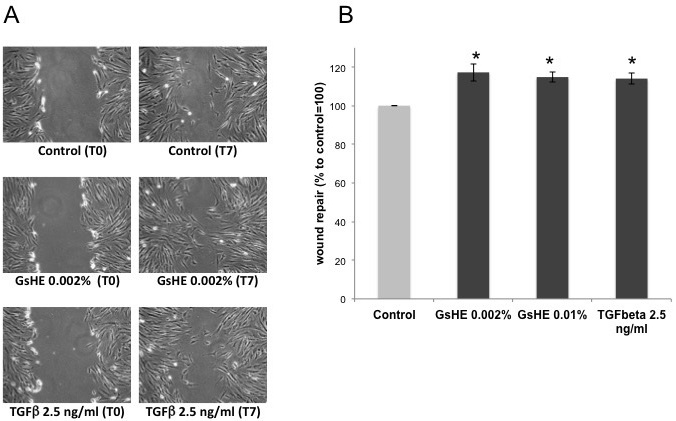
(A) Photographs of the cultures just after the scratching (T0), and after 7 h from the scratching (T7). The relative treatment is indicated below each picture. (B) The cell-free areas at T0 and T7 for each treatment were measured and compared, and the values expressed as percentages to the control, arbitrarily set as 100%. The error bars represent standard deviations, and the asterisks indicate statistically significant measures (*p<0.01; **p<0.001).
GsHE Regulates Sebum Production in Vivo
On the basis of the results obtained from the in vitro studies, we employed the extract of G. sulphuraria in a clinical sebum normalizing efficacy test, performed on 20 male and female volunteers, aged from 18 to 50, having a greasy face skin. Twice a day, for 30 consecutive days, the volunteers applied a cream containing 0.002% of GsHE on the left side of the face and an emulsion without GsHE on the right side of the face. The concentration of 0.002% was chosen because it was the one giving the best results in most of the cell based assays. As control, the forehead remained untreated for the entire duration of the study and the formula used in the tests is reported in Table 1.
Table 1: Cosmetic formula used in the clinical tests on sebum normalizing activity
|
CAS
|
INCI Name |
%INC
|
| 7732-18-5 |
AQUA (WATER) |
83.552 |
| 73398-61-5 |
CAPRYLIC/CAPRIC TRIGLYCERIDE |
4.600 |
| 57-11-4 |
STEARIC ACID |
3.300 |
| 31566-31-1 |
GLYCERYL STEARATE |
2.400 |
| 9004-99-3 |
PEG-100 STEARATE |
1.700 |
| 67762-27-0 |
CETEARYL ALCOHOL |
1.050 |
| 36653-82-4 |
CETYL ALCOHOL |
1.000 |
| 122-99-6 |
PHENOXYETHANOL |
0.800 |
| 56-81-5 |
GLYCERIN |
0.490 |
| 9004-99-3 |
PEG-20 STEARATE |
0.350 |
| 70445-33-9 |
ETHYLHEXYGLYCERIN |
0.300 |
| 76050-42-5 |
CARBOMER |
0.260 |
| 139-33-3 |
DISODIUM EDTA |
0.100 |
| 1310-73-2 |
SODIUM HYDROXIDE |
0.096 |
| 68917-51-1 |
GsHE (ABSENT IN PLACEBO) |
0.002 |
|
|
100.000 |
As showed in Figure 5, the mean cutaneous sebum amount was significantly decreased by GsHE treatment: the sebum excretion decreased by an average value of 58.5% after 15 days and 75.71% after 30 days of application with respect to the mean value measured at T0; the variations were extremely statistically significant (p<0.0001). A significant decrease in the sebum level was observed also in the placebo treatment after 30 days, but it was more limited and corresponded to approximately half of that observed with GsHE application. The mean sebum value did not change in the forehead treatments (control).
Figure 5: Clinical test.
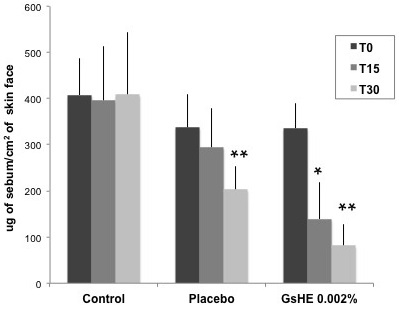
20 panelists applied a cream without the active (placebo) and a cream containing GsHE (GsHE 0.002%), respectively on the right and left half face, twice a day for 28 consecutive days. After 15 days (T15) and 30 days (T30) of application, the sebum level on the skin was measured by the instrument Sebumeter® SM815, and compared with that measured in untreated skins (Control). The values were reported as micrograms of sebum per cm2 of skin at the 3 different times of measure. Asterisks indicate significant differences (*p<0.01; **p<0.001).
This results confirm that GsHE can be an effective cosmetic ingredient, particularly suitable to relieve the problems associated with sebum overproduction in oily skins.
CONCLUSION
Research on microalgae has highlighted the huge applicative potentiality of these groups of microorganisms in industry: they are extremely diversified and phylogenetically distant one from the other, which makes them a natural inexhaustible source of unexplored compounds with the most various and divergent industrial applications, ranging from food, cosmetics, detergent to biofuel. Among the microalgae, the extremeophiles are even more attracting in terms of the applicative outputs of the molecules they synthesize: they have adapted to survive in extremely harsh environments, thus they represent highly effective biofactories of novel chemicals with unknown biological functions.
Thanks to their peculiarities, extremeophile microalgae have drawn the attention of researchers in the fields of Dermatology and Cosmetics, and some ingredients, derived from these species, have already found applications in the skin care market23 and used by many top cosmetic brands. The species Dunaliella salina, living in high salinity environments, is the most used source of β-carotene, that is one of the leading antioxidant compounds employed in skin care formulas.24 In addition, several types of extracts from Arthrospira maxima, commonly known as Spirulina, a cyanophyte living in alkaline waters, are claimed to repair the signs of early skin aging, exert a tightening effect and have wound healing properties, thanks to the presence of the protein C-phycocyanin.25,26 Recently, an extract deriving from the snow micro alga Chlamydocapsa was also developed as product to fight extrinsic and intrinsic skin aging.27
The extremeophilic red alga Galdieria sulphuraria lives in hot, sulfur-rich, acidic environments and shows an enormous metabolic flexibility, growing either photo autotrophically or heterotrophically on more than 50 carbon sources. It is also able to change its metabolism and its storage products according to the different growth conditions.28 As extremophile, Galdieria is remarkably flexible because it has acquired a large number of genes from bacteria, and integrated them into a functional network.29 Thanks to this evolutionary process, the species Galdieria has developed unique properties and adaptations due to the production of specific secondary metabolites. Some of these metabolites have been identified by a recent study which demonstrated the synthetic capacity of Galdiera to produce a class of compounds, the galactosylglycerols, specifically involved in the acclimation to various abiotic stresses, especially high salinity. Among them, the main photosynthetic products floridside and isoforidoside are known also to contribute to the osmotic stress resistance of cells.30
In the present study, we investigated the potential use of the microalga G. sulphuraria in skin care and, by performing both cellular assays in vitro and clinical tests, we demonstrated that the extract obtained from G. sulphuraria was able to reduce 5αR1 expression in fibroblasts and enhance the skin’s natural defense response by upregulating hBD2 expression in keratinocytes, resulting in a significant reduction of sebum production in the skin. Thanks to these activities, GsHE represents an attractive active ingredient for the skin care market, in particular as adjuvant in products designed to fight greasy skins, or even those affected by seborrheic dermatitis or acne. The extract was also capable of enhancing the healing process, as demonstrated by the cell culture tests, reinforcing the role of the extract as active ingredient in formulas addressed to alleviate the outbreak of lesions typical of acneic skins. Interestingly, these activities can be easily exploited in the hair care market as well, which is always looking for new and safe ingredients having a sebum reducing effect on the scalp, formulated either in conditioners or shampoos.31,32
ACKNOWLEDGEMENT
We want to thank the professors A. Pollio and G. Pinto, University of Naples Federico II, Italy, for providing the microalga strain for the studies. The research was supported by the grants “Campus Bioframe” – POR CAMPANIA FESR2007 2013, and “Campania Bioscience” -PON03PE_00060_3.
CONFLICTS OF INTEREST
None.