INTRODUCTION
Zoonotic diseases, those which are spread from animals to humans, are estimated to comprise 60% of all diseases afflicting the humans today.1 Rabies is a zoonotic disease affecting public health, causing about tens of thousands of human deaths annually, such that a majority of the cases are reported particularly among children belonging to the rural parts of developing countries.2 There are multiple viruses from the genus Lyssavirus and family
Rhabdoviridae that are capable of causing rabies. The viruses associated with this disease include rabies virus, Australian bat lyssavirus and European bat lyssavirus. Rabies virus is transmitted through the saliva of an infected animal when it comes in contact with the mucous membrane or penetrates the skin through scratches and bite wounds.3,4 It has also been documented to spread less commonly through organ transplant infections.5,6 Incubation time lasts on an average for about five weeks; however, it can range from a few days to several years. Incubation time varies depending on several viral and host factors including the viral load, the path of viral entry and the proximity of the wounds to the brain. Clinical symptoms of the disease may be implicated as hypersensitivity, increased irritability, aggression, aerophobia and hydrophobia, hypersalivation, vocalization, fever, convulsions and paralysis.
Rabies virus is transmissible to all mammals and members of the order Carnivora and Chiroptera, therefore considered as the main reservoir hosts. Canines are the predominant reservoir hosts for the rabies virus globally and are responsible for 99% of the human infections.2,7 The risk for human transmission from dogs is greatest in Africa and Asia, followed by Latin America.2
Rabies is endemic to the island of Grenada and had been clinically suspected as far back as 1902. The first laboratory confirmed case of rabies was reported in 1952 in a Bovine species.8 Small Indian mongoose (Herpestes auropunctatus) were introduced in 27 Caribbean countries including Grenada to control rats and snakes in sugarcane plantations, in the late 19th century.9 Until 1956, rabies was not laboratory confirmed in mongooses in Grenada, till the time that rabies was diagnosed in a mongoose found dead on the road. Several studies examining rabies in Grenada was conducted nearly 40 years ago and identified mongooses, canines, bovines and felines as the predominantly infected species.10 The current study is a passive re-examination of the occurrences, patterns, and distribution of rabies infections in animals in Grenada over the last 16 years (2001-2016) to identify targets for preventive care.
MATERIALS AND METHODS
Grenada is a tri-island country located between the Atlantic Ocean and the Caribbean Sea, roughly 100 miles north of Venezuela. The island is separated into 6 different parishes, St. Patrick, St. Mark, St. Andrew, St. John, St. George, and St. David. St. George’s University is located at the southernmost tip of the island in the second largest parish, St. George. Cases of animal species suspected for rabies were submitted through SGU small animal hospital, private veterinarians, the animal control unit of Ministry of Health, farmers and pet owners to the St. George’s University, School of Veterinary Medicine, Pathobiology Department for rabies diagnosis. All cases submitted between March 2001 and July 2016 were archived from the record of the diagnostic laboratory and included in this study. Over 3,000 cases of animals submitted for necropsy were reviewed and data were collected if the animal was suspected or tested positive for rabies. Data included the species, sex, age, location, clinical signs, vaccination status, test date and the laboratory tests performed for each animal.
In diagnostic laboratory of the School of Veterinary medicine, St. George’s University following techniques were employed on the brain tissue for rabies diagnosis;
Histopathology of brain tissue: Cross section of the brain stem and cerebellum was fixed in 10% formalin, processed through paraffin embedding and stained by hematoxylin and eosin. Sections were examined under light microscope for the presence of inclusion bodies in the cytoplasm of neurons. Inclusions were observed bright red in bluish background.
Fluorescent antibody test: Impression smears of cut section of brain stem and the cerebellum were made, fixed in cold acetone and stained with a cocktail of three fluorescein-labeled monoclonal antibodies directed against the nucleocapsid (N) protein of rabies virus manufactured by light diagnostics Rabies DFA reagent, Millipore,
Livingston, UK. Slides were viewed under the fluorescent microscope (Nikon Eclipse 80 fluorescence. attachment). Rabies positive smears gave apple color fluorescence.
Polymerase chain reaction (PCR): This was followed as described by Zieger et al.9 Total RNA was extracted from 30-50 mg homogenized brain tissue (brain stem and cerebellum) using Trizol reagent (Invitrogen, Grand Island New York, USA.). The RT-PCR one step iScript was used on 200-600 ng total RNA per 50 microliter reaction mix. The primers used for amplification targeted a 110 bp fragment of the highly conserved region of the nucleoprotein (N) gene. Primers had following sequence: JW12 5’ATGTAACACCYCTACAATG3’ and N 165-146 5-’GCAGGGTAYTTRACTCATA-3’. The RT-PCR and amplification reactions were performed in an Eppendorf Master cycle ProS (Eppendorf AG, Hamburg, Germany). PCR products were visualized using and ethidium-bromide stained 3% agarose gel electrophoresis.
RESULTS
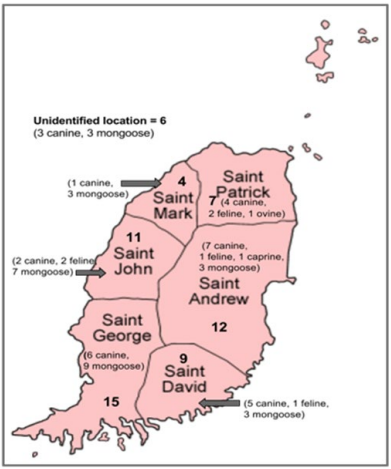
Between March 2001 and July 2016, a total of 173 animals, from 8 different species (91 canines, 46 mongooses, 16 felines, 9 bats, 5 caprines, 3 opposums, 2 ovines, and 1 bovines), were tested for rabies at the St. George’s University, School of Veterinary Medicine, Grenada. A total of 64 animals were tested positive for rabies virus out of the 173 tested which included canine (28/91 i.e. 30.76%), mongoose (28/46 i.e. 60.86%), feline (6/16 i.e. 37.5%) ovine (1/2 i.e. 50%) and caprine (1/5 i.e. 20%) (Figure 1). No positive cases were reported in the bats, opposums or bovines. Figure 1 depicts the number of animals tested and the number of positive results for each species. Out of the 173 animals tested, 21 were excluded from the study either because of incomplete submission records,9 inconclusive results,9 or non diagnostic samples.3 The sex of the tested and positive animals between males (31/64) and females (25/64) is included in Table 1. Eight cases, five of which were positive, did not have their sex listed. Yearly distribution of rabies cases have been shown in Figure 2. Majority of the positive cases of rabies were observed in adult animals (47/123), with only a few found in juveniles (8/33), although some did not have their ages listed (9/17). All six parishes reported positive cases for rabies; St. Andrew12, St. George15, St. John11, St. David9, St. Patrick7, and St. Mark4 (Figure 3, Table 2). Commonly reported clinical symptoms included a change in behavior, aggression with unprovoked biting, hypersalivation and dysphagia, ataxia and weakness. Additionally, vomiting, pawing at the face and the inability to close the mouth were also reported. Rarely, dyspnea, nystagmus, muscle tremors were also reported. None of the animals affected by rabies were reported as vaccinated, they were either not vaccinated or their vaccination status was unknown. Only two of the negatively reported cases were indicative of up to date vaccinations. Out of 173 cases tested, 107 were performed during the dry season (December-June) and 66 were performed during the rainy season (July-November). Out of 64 cases that tested positive for rabies, 37 occurred in the dry season (58%) and 27 occurred in the rainy season (42%). Three tests were used to identify animals as positive or negative for rabies infection: Direct fluorescent antibody testing (DFAT), polymerase chain reaction (PCR), and histopathology. A breakdown of the tests used to identify positive cases from each species is shown in Table 3. A combination of DFAT and PCR were the most commonly used method, thereby becoming an almost exclusive technique of diagnosis 2013 onwards. Nine samples examined by histopathology alone were considered as inconclusive based on the clinical implications of meningoencephalitis without a confirmation of the presence of Negri bodies. The collected data showed a positive trend for a number of animals tested as well as the number of animals testing positive over the 16 year period (Figure 2). The greatest number of animals both tested for and positive for rabies was in 2013.
Figure 1: Rabies Cases by Species (2001-2016).
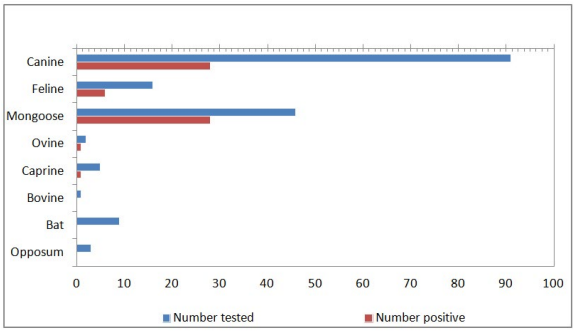
Figure 2: Rabies Cases from March 2001 to July 2016.
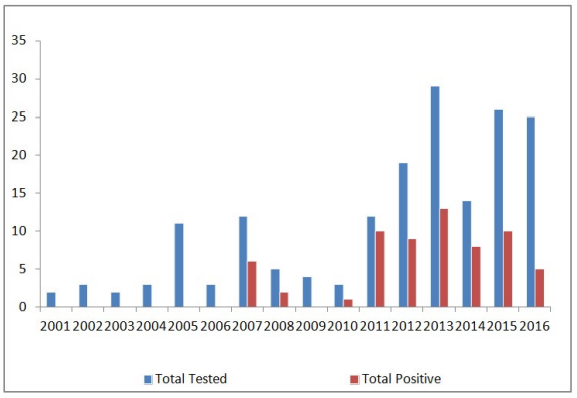
Figure 3: Positive Rabies Cases (2001-2016) in Various
Parishes of Grenada.
| Table 1: Positive Species Identified by Sex. |
|
Species
|
Male |
Female |
Unknown |
Total |
| Canine |
21 |
7 |
– |
28
|
|
Feline
|
1 |
4 |
1 |
6 |
| Mongoose |
8 |
13 |
7 |
28
|
|
Ovine
|
1 |
– |
– |
1 |
| Caprine |
– |
1 |
– |
1
|
|
Total
|
31 |
25 |
8 |
64
|
| Table 2: Positive Cases by Species and Location. |
|
Parish
|
Canine |
Feline |
Mongoose |
Ovine |
Caprine |
Total |
| Saint Patrick |
4 |
2 |
– |
1 |
– |
7
|
|
Saint Mark
|
1 |
– |
3 |
– |
– |
4 |
| Saint Andrew |
7 |
1 |
3 |
– |
1 |
12
|
|
Saint John
|
2 |
2 |
7 |
– |
– |
11 |
| Saint George |
6 |
|
9 |
– |
– |
15
|
|
Saint David
|
5 |
1 |
3 |
– |
– |
9 |
Unidentified
location |
3 |
– |
3 |
– |
– |
6
|
|
Total
|
28 |
6 |
28 |
1 |
1 |
64
|
| Table 3: Test Methods Used to Identify rabies Positive Cases (64 Total). |
|
Species
|
PCR |
Histopathology |
DFAT+PCR |
DFAT+Histopathology |
DFAT+PCR+Histopathology |
| Canine |
– |
6 |
11 |
1
|
10 |
|
Feline
|
– |
– |
4 |
– |
2 |
| Mongoose |
1 |
1 |
23 |
1
|
2 |
|
Ovine
|
– |
– |
1 |
– |
– |
| Caprine |
– |
– |
– |
–
|
1 |
|
Total
|
1 |
7 |
39 |
2
|
15 |
DISCUSSION
Previously published reports on clinical surveys of rabies in Grenada from 1952 show that among the domestic species, bovine and canines were primarily affected,11 however it is believed that reported cases for canines in Grenada have been declining in terms of diagnosis with the institution of vaccination clinics,10,12 The previous survey reports indicated annual variation in the number of rabies positive cases in various species of animals.11 No explanation for this variation is found in the reports. In the current survey (2001-2016), up to 2006, no rabies positive animals were observed. However, a large number of animals were diagnosed positive for rabies during 2011 to 2016. This increase in the number of identified positive cases could be attributed to the application of reliable diagnostic procedures in the laboratory to test for rabies, established since 2011 at the St. George’s University. Before 2010, this diagnosis was based primarily on the histopathology of the brain tissue.
In an extensive epidemiological study of rabies in mongoose, conducted in Grenada during the 1960’s and 1970’s, over half of the human population was exposed to rabies as a result of contact with a mongoose.10 Data on human exposure and administration of post exposure prophylaxis could not be acquired for the current study. However, in this study, 16 reports (8 canines, 5 felines, 3 mongooses) indicated animals testing positive for rabies to be attacking and biting humans. Since the mongoose is a wildlife species, they are less likely than domestic species and family pets, to be caught and brought in for testing. Although less likely to bite when rabid, ruminants can still transmit the virus to humans through direct contact with their saliva.5 The lower number of reported cases in this study involving ruminants makes the interpretation of previous data difficult.
Six species of bats including Artibeas jamaicensis have been tested for rabies in Grenada during 1968-1977; one bat (in Artibeas jamaicensis) was found positive by FAT and mouse inoculation test. Rabies neutralizing antibodies were detected in all six species tested with 40.55% in A. jmaicensis.13 However, since there are no reports of vampire bats in Grenada, the role of other species of bats in Grenada is not known in rabies transmission to humans.8,13 Only 7 bats were reported in this study, all of which were negative for rabies virus as identified with DFAT and confirmed with PCR. One case of rabies was reported in a Didelphis opossum in 1969.14 Three opossums, another wildlife species in Grenada, were also included in this study and were diagnosed negative by DFAT and confirmed with PCR. More studies need to be conducted with larger sample sizes before bats and opossums can be ruled out as a reservoir or host species for rabies in Grenada.
Although, there is a slight discrepancy in positive cases reported between the dry (58%) and wet (42%) seasons, the significance of this discrepancy needs to be determined. An increase in canine rabies has been observed in North and South America during the spring months in coordination with the mating season and the close interaction between dogs.15 Grenada’s climate is tropical with minimal fluctuations in temperature and decrease in rainfall seen during the months of December to June. It is believed that the lack of temperature variability leads to the disruption of typical breeding seasons in canines in Grenada. However, the hypothesis needs to be confirmed.
The laboratory methods used for the diagnosis of positive rabies case are presented in Table 3. The results indicate that use of DFAT and PCR were most sensitive. The use of DFAT and PCR on all rabies suspected cases is recommended for the rabies diagnosis.
The majorities of dogs on the island are free-roaming or are kept tied outside. In the present survey, a higher incidence of rabies in male dogs was recorded. Further study is warranted to identify the cause of higher incidence of rabies in male dogs. Free-roaming dogs or dogs kept outside are more likely to come in contact with humans. These dogs are also more likely to come in contact with wildlife species, in particular mongoose, which act as host reservoirs for the disease. Contrary to dogs, in this study, more female mongoose was tested positive for rabies. Further study on the behavior of mongoose may explain the higher incidence of female positivity of rabies in this species.
Stray dog control programs, in coordination with vaccination programs, have been helping to decrease canine rabies reservoirs in many Latin American countries since the 1980’s.16,17 Current programs of rabies control in Grenada include veterinary and government facilitated vaccination clinics for animals, education of individuals and children, pre-exposure vaccination of the risk individuals, and post-exposure prophylaxis for humans. However, a regular surveillance system to report the suspected cases of rabies in human and animals is essential for the prevention and control of this fatal disease.
ACKNOWLEDGEMENTS
The authors are grateful to the technical staff of the Veterinary Diagnostic Laboratory, School of Veterinary Medicine, St. George’s University, Grenada, West Indies, for helping in data retrieval.
COMPETING INTEREST
The authors declare that there is no competing interest.