INTRODUCTION
Bovine trypanosomiasis is a parasitic disease caused by Trypanosoma (Dutonella) vivax that mainly affects ungulates, including cattle, sheep, goats, horses, camels and various species of wild antelope and buffalo.1,2
In West Africa, T. vivax is an important and pathogenic trypanosome in cattle, being a significant disease to animal breeding transmitted by the tsetse fly (Glossina spp). In Latin America, mechanical vectors transmit the parasite.3,4 In Brazil, T. vivax was first identified in a buffalo in Pará state, subsequently to an outbreak of trypanosomiasis in Pantanal affecting 10 of 29 head of cattle.
Symptoms depend on the degree of the disease.5 Often is observed hyper acute infection, characterized by severe anemia, bleeding in mucous surface and thrombocytopenia arising.6 Acute infection, which generates septicemia with fever and marked parasitemia two weeks after infection, may result in death.7 It is possible to find ecchymotic hemorrhages,5 intermittent fever, swollen lymph nodes, tachycardia, poor body condition, decreased fertility,8,9 leukopenia, watery eyes, progressive weakness, abortions9 and anemia without hemoglobinuria.5
Observing fresh blood under microscope is a direct method adequate to diagnose the acute disease.5 In contrast, the chronic phase of the disease is diagnosed by detection of antibodies against T. vivax using the indirect immunofluorescence.10,11
This study aimed to perform a retrospective investigation of cattle with T. vivax n Veterinary Hospital of Uberaba-Brazil.
MATERIAL AND METHODS
A retrospective study was performed with 285 bovine from Uberaba (152 animals), Prata (90 animals), Pirajuba (20 animals), Campo Florido (7 animals), Veríssimo (6 animals), Comendador Gomes (4 animals), Monte Alegre de Minas (3 animals), Frutal (2 animals) and Coromandel (1 animal) that had samples forwarded to Hospital Veterinário de Uberaba from May, 2012 to September, 2013. The animals were separated according to gender being 47 males, 219 females and 19 did not have specified gender. The bovines also had different breeds, being Holstein (4 animals), Gyr (8 animals), Girolando (180 animals) and undefined cattle breed (93 animals). In addition, the animals were also separated according to ages.
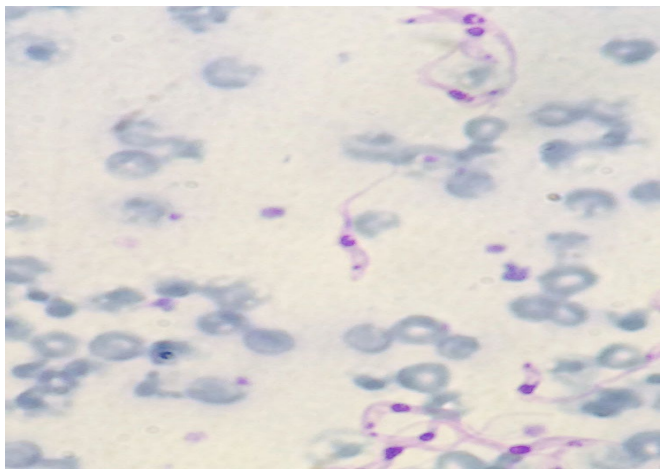
The protozoan was detected by Buffy coat technique that consists in centrifuging the blood to be analyzed in a closed hematocrit micro tube. After centrifugation, the tube is broken between cellular and liquid part and a small portion of material is deposited on the slide, according to the technique described.11 The slides were stained with Quick Panoptic® kit and visualized in an optical microscope at 100X objective with immersion oil. The animals were considered positive when was possible to observe trypomastigote form (Figure 1). The data were analyzed by descriptive statistics.
Figure 1: Positive animal for Trypanosoma vivax using smear made by Buffy coat technique (trypomastigote form). 100X magnification stained with quick Panoptic®.
RESULTS AND DISCUSSION
The Triângulo Mineiro region is located in state of Minas Gerais – Brazil. This region is one of the most important sites for the production of cattle and exports genetic material to different places around world and there is little study about this parasitosis in Minas Gerais state.
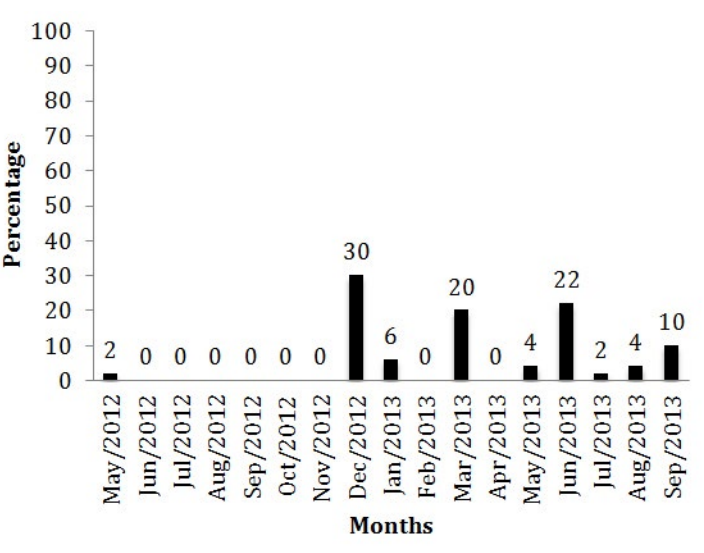
The T. vivax occurrence in different months of the year is shown in figure 2. The protozoan epidemiology is directly related to the rainfall period.4,11,12 Recent studies13 analyzed the positivity percentage of animals from Uberaba found 16.2% of positivity when searching for antibodies anti-T. vivax, value that was near to what was found in the present study.
Figure 2: Percentage of positive and negative animals to T. vivax in different months.
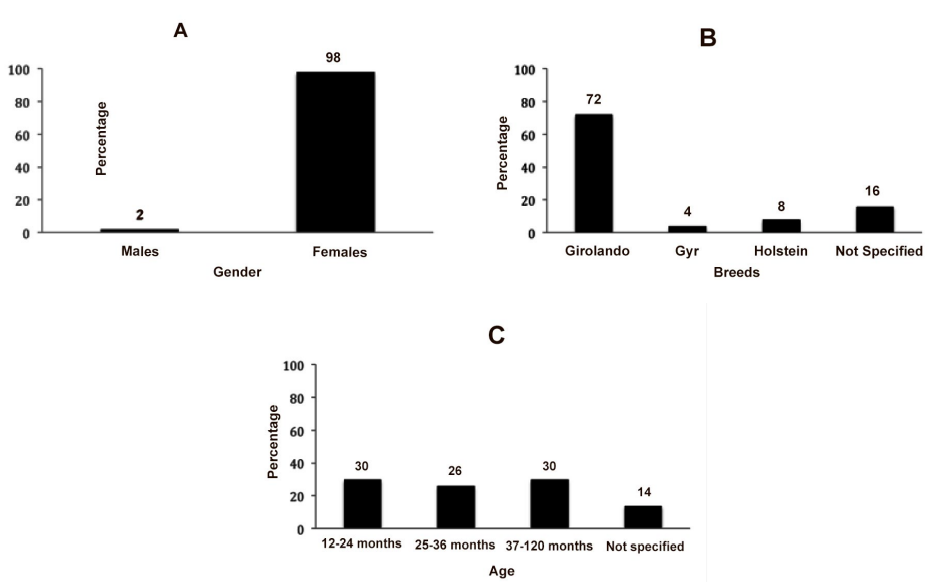
Males showed 2% (1) positivity, lowered when compared to females 98% (49) (Figure 3A). The highest T. vivax occurrence in females is explained firstly because of the stress to which they are submitted,14 i.e. Gestation, lactation, and secondly because females are more likely to contamination with fomites i.e. when the same needle is used for many animals in application of medication such as oxytocin before milking.
When the T. vivax occurrence in the region of Uberaba and adjacent regions was studied,13 was shown that in 91.6% of positive properties for T. vivax the needle exchange between animals while administrating drugs was not done.
In the present study Girolando animals had 72% (36) positivity followed by not specified breeds 16% (8), Holstein breed, which had 8% positivity (4). Gyr breed had 4% (2) positivity (Figure 3B). The first trypanosomiasis report in dairy herd for the southwest region was described in recent studies15 in the state of São Paulo, reporting animals breed Holstein and Girolando. In the state of Minas Gerais the first report was done in 2008.16 Other breeds were described with infection such as Brahman.17 Some breed developed what is called trypanotolerancy, which is the resistance to the infection and keep in the condition of asymptomatic carrier.18
The fact that the animals from the present study are in the most part dairy cattle is because the region of Minas Gerais is a pole in milk production, having big dairy herds and being the main state in milk production in Brazil.19
The diagnosis in dairy cattle is easier, once the milk production is hampered,4 being promptly noticed for those who are handling the animals daily.
The animals were separated in groups according the age, being 30% (15) older than 12 months, 26% (13) between 25 to 36 months, 30% (15) between 37 to 120 months and14% (7) did not have the age identified (Figure 3 C).
Figure 3: A – Percentage of positive and negative animals to T. vivax in males and females; B – Occurrence of positive animals for T. vivax among the different breeds; C – Percentage of positive animals among different ages.
The resistance to the protozoan besides other factors can vary with age.14 This could be explained because younger animals do not have developed immunity to the pathology, being the first contact within the first months of life, what lead to acute cases that were detectable to the buffy coat method.
It is important to emphasize that the Buffy coat technique allows detecting circulating parasite, i.e., in the acute phase or when the animal undergoes immunosuppression. Much of infections in cattle from endemic areas tend to be chronic course and not always fatal.20
In South America, T. vivax transmission is mechanical. This work shows that even without the presence of the tsetse fly, there are bovine trypanosomiasis cases in Brazil, with severe symptoms attributed mainly to Tabanidae. In addition, the mechanical transmission by fomites is relatively easy. Brazil is an important pole in the production chain of animals. For this reason attention should be taken aiming the control of this agent in farms of the country.21
CONFLICTS OF INTEREST: None.